Stress Disrupts Human Hippocampal-Prefrontal Function during Prospective Spatial Navigation and Hinders Flexible Behavior
- PMID: 32243859
- PMCID: PMC7331937
- DOI: 10.1016/j.cub.2020.03.006
Stress Disrupts Human Hippocampal-Prefrontal Function during Prospective Spatial Navigation and Hinders Flexible Behavior
Abstract
The ability to anticipate and flexibly plan for the future is critical for achieving goal-directed outcomes. Extant data suggest that neural and cognitive stress mechanisms may disrupt memory retrieval and restrict prospective planning, with deleterious impacts on behavior. Here, we examined whether and how acute psychological stress influences goal-directed navigational planning and efficient, flexible behavior. Our methods combined fMRI, neuroendocrinology, and machine learning with a virtual navigation planning task. Human participants were trained to navigate familiar paths in virtual environments and then (concurrent with fMRI) performed a planning and navigation task that could be most efficiently solved by taking novel shortcut paths. Strikingly, relative to non-stressed control participants, participants who performed the planning task under experimentally induced acute psychological stress demonstrated (1) disrupted neural activity critical for mnemonic retrieval and mental simulation and (2) reduced traversal of shortcuts and greater reliance on familiar paths. These neural and behavioral changes under psychological stress were tied to evidence for disrupted neural replay of memory for future locations in the spatial environment, providing mechanistic insight into why and how stress can alter planning and foster inefficient behavior.
Keywords: Cognitive Control; Hippocampus; Memory; Navigation; Stress; fMRI.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures

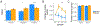




Comment in
-
Cognitive Neuroscience: Why Do We Get Lost When We Are Stressed?Curr Biol. 2020 May 18;30(10):R439-R441. doi: 10.1016/j.cub.2020.03.065. Curr Biol. 2020. PMID: 32428474
References
-
- Buckner RL, and Carroll DC (2007). Self-projection and the brain. Trends Cogn. Sci 11, 49–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

