Estrogen-dependent hypersensitivity to diabetes-evoked cardiac autonomic dysregulation: Role of hypothalamic neuroinflammation
- PMID: 32243927
- PMCID: PMC7202046
- DOI: 10.1016/j.lfs.2020.117598
Estrogen-dependent hypersensitivity to diabetes-evoked cardiac autonomic dysregulation: Role of hypothalamic neuroinflammation
Abstract
Aims: To investigate if autonomic dysregulation is exacerbated in female rats, subjected to diabetes mellitus (DM), via a paradoxical estrogen (E2)-evoked provocation of neuroinflammation/injury of the hypothalamic paraventricular nucleus (PVN).
Main methods: We measured cardiac autonomic function and conducted subsequent PVN neurochemical studies, in DM rats, and their respective controls, divided as follows: male, sham operated (SO), ovariectomized (OVX), and OVX with E2 supplementation (OVX/E2).
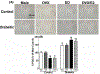
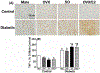
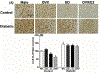
Key findings: Autonomic dysregulation, expressed as sympathetic dominance (higher low frequency, LF, band), only occurred in DM E2-replete (SO and OVX/E2) rats, and was associated with higher neuronal activity (c-Fos) and higher levels of TNFα and phosphorylated death associated protein kinase-3 (p-DAPK3) in the PVN. These proinflammatory molecules likely contributed to the heightened PVN oxidative stress, injury and apoptosis. The PVN of these E2-replete DM rats also exhibited upregulations of estrogen receptors, ERα and ERβ, and proinflammatory adenosine A1 and A2a receptors.
Significance: The E2-dependent autonomic dysregulation likely predisposes DM female rats and women to hypersensitivity to cardiac dysfunction. Further, upregulations of proinflammatory mediators including adenosine A1 and A2 receptors, TNFα and DAPK3, conceivably explain the paradoxical hypersensitivity of DM females to PVN inflammation/injury and the subsequent autonomic dysregulation in the presence of E2.
Keywords: Adenosine; Autonomic dysregulation; Diabetes; Estrogen; Hypothalamus.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no competing interests.
Figures





Similar articles
-
Aerobic training normalizes autonomic dysfunction, HMGB1 content, microglia activation and inflammation in hypothalamic paraventricular nucleus of SHR.Am J Physiol Heart Circ Physiol. 2015 Oct;309(7):H1115-22. doi: 10.1152/ajpheart.00349.2015. Epub 2015 Aug 7. Am J Physiol Heart Circ Physiol. 2015. PMID: 26254332
-
Preautonomic neurons in the paraventricular nucleus of the hypothalamus contain estrogen receptor beta.Brain Res. 2003 Jun 13;975(1-2):99-109. doi: 10.1016/s0006-8993(03)02594-0. Brain Res. 2003. PMID: 12763597
-
Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus.Neuroscience. 2009 Mar 17;159(2):883-95. doi: 10.1016/j.neuroscience.2008.12.058. Epub 2009 Jan 7. Neuroscience. 2009. PMID: 19166915 Free PMC article.
-
The paraventricular nucleus and heart failure.Exp Physiol. 2014 Feb;99(2):332-9. doi: 10.1113/expphysiol.2013.072678. Epub 2013 Dec 6. Exp Physiol. 2014. PMID: 24317407 Review.
-
Cardiac Neuroanatomy and Fundamentals of Neurocardiology.Card Electrophysiol Clin. 2024 Sep;16(3):229-237. doi: 10.1016/j.ccep.2024.01.002. Epub 2024 Mar 4. Card Electrophysiol Clin. 2024. PMID: 39084716 Review.
Cited by
-
Protein Kinases Mediate Anti-Inflammatory Effects of Cannabidiol and Estradiol Against High Glucose in Cardiac Sodium Channels.Front Pharmacol. 2021 Apr 28;12:668657. doi: 10.3389/fphar.2021.668657. eCollection 2021. Front Pharmacol. 2021. PMID: 33995099 Free PMC article.
-
Estrogen dampens central cannabinoid receptor 1-mediated neuroexcitation and pressor response in conscious female rats.Biochem Pharmacol. 2022 Jul;201:115102. doi: 10.1016/j.bcp.2022.115102. Epub 2022 May 23. Biochem Pharmacol. 2022. PMID: 35617998 Free PMC article.
-
Estrogen-mediated mitigation of cardiac oxidative stress in ovariectomized rats is associated with upregulated cardiac circadian clock Per2 and heart-specific miRNAs.Life Sci. 2023 Oct 15;331:122038. doi: 10.1016/j.lfs.2023.122038. Epub 2023 Aug 22. Life Sci. 2023. PMID: 37619835 Free PMC article.
-
Heart Failure in Menopause: Treatment and New Approaches.Int J Mol Sci. 2022 Dec 1;23(23):15140. doi: 10.3390/ijms232315140. Int J Mol Sci. 2022. PMID: 36499467 Free PMC article. Review.
-
Estrogen receptor beta activity contributes to both tumor necrosis factor alpha expression in the hypothalamic paraventricular nucleus and the resistance to hypertension following angiotensin II in female mice.Neurochem Int. 2022 Dec;161:105420. doi: 10.1016/j.neuint.2022.105420. Epub 2022 Sep 25. Neurochem Int. 2022. PMID: 36170907 Free PMC article.
References
-
- Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:816–23. - PubMed
-
- Collin B, Busseuil D, Zeller M, Perrin C, Barthez O, Duvillard L, et al. Increased superoxide anion production is associated with early atherosclerosis and cardiovascular dysfunctions in a rabbit model. Mol Cell Biochem. 2007;294:225–35. - PubMed
-
- Deng X, Feng X, Li S, Gao Y, Yu B, Li G. Influence of the hypothalamic paraventricular nucleus (PVN) on heart rate variability (HRV) in rat hearts via electronic lesion. Biomedical materials and engineering. 2015;26 Suppl 1:S487–95. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

