BTK inhibitor therapy is effective in patients with CLL resistant to venetoclax
- PMID: 32244251
- PMCID: PMC7316215
- DOI: 10.1182/blood.2020004782
BTK inhibitor therapy is effective in patients with CLL resistant to venetoclax
Abstract
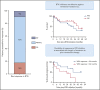
Highly active BTK inhibitors (BTKis) and the BCL2 inhibitor venetoclax have transformed the therapeutic landscape for chronic lymphocytic leukemia (CLL). Results of prospective clinical trials demonstrate the efficacy of venetoclax to salvage patients with disease progression on BTKis, but data on BTKi therapy after disease progression on venetoclax are limited, especially regarding durability of benefit. We retrospectively evaluated the records of 23 consecutive patients with relapsed/refractory CLL who received a BTKi (ibrutinib, n = 21; zanubrutinib, n = 2) after stopping venetoclax because of progressive disease. Median progression-free survival (PFS) and median overall survival after BTKi initiation were 34 months (range, <1 to 49) and 42 months (range, 2-49), respectively. Prior remission duration ≥24 months and attainment of complete remission or undetectable measurable residual disease on venetoclax were associated with longer PFS after BTKi salvage (P = .044 and P = .029, respectively). BTKi therapy achieved durable benefit for patients with the BCL2 Gly101Val venetoclax resistance mutation (estimated 24-month PFS, 69%). At a median survivor follow-up of 33 months (range, 2-53), 11 patients remained on BTKi and 12 had stopped therapy because of disease progression (n = 8) or toxicity (n = 4). Our findings indicate that BTKi therapy can provide durable CLL control after disease progression on venetoclax.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: A.W.R., M.A.A., T.E.L., and V.S.L. are employees of the Walter and Eliza Hall Institute of Medical Research, which receives milestone and royalty payments related to venetoclax. A.W.R., M.A.A., and T.E.L. are recipients of a share in royalty payments paid to the Walter and Eliza Hall Institute of Medical Research. S.M.H. has received honoraria from Gilead and nonfinancial assistance from AbbVie. C.S.T. has received honoraria and research funding from AbbVie and Janssen and honoraria from BeiGene. A.W.R. has received research funding from AbbVie, Genentech, Servier, Janssen, and BeiGene. J.F.S. receives research funding from AbbVie, Genentech, Celgene, and Janssen and is an advisory board member for and has received honoraria from AbbVie, Acerta, Celgene, Genentech, Janssen, Roche, Sunesis, and Takeda. M.A.A. has received honoraria from AbbVie and CSL Behring. T.E.L. has received honoraria from AbbVie. The remaining authors declare no competing financial interests.
Figures

Comment in
-
Inverting the BTK-BCL2 order.Blood. 2020 Jun 18;135(25):2205-2207. doi: 10.1182/blood.2020005886. Blood. 2020. PMID: 32556131 Free PMC article.
Similar articles
-
Is there a role for anti-CD20 antibodies in CLL?Hematology Am Soc Hematol Educ Program. 2021 Dec 10;2021(1):68-75. doi: 10.1182/hematology.2021000234. Hematology Am Soc Hematol Educ Program. 2021. PMID: 34889417 Free PMC article.
-
Real-world comparison of health care costs of venetoclax-obinutuzumab vs Bruton's tyrosine kinase inhibitor use among US Medicare beneficiaries with chronic lymphocytic leukemia in the frontline setting.J Manag Care Spec Pharm. 2024 Oct;30(10):1106-1116. doi: 10.18553/jmcp.2024.24049. Epub 2024 Jul 24. J Manag Care Spec Pharm. 2024. PMID: 39046941 Free PMC article.
-
Bruton's tyrosine kinase inhibition increases BCL-2 dependence and enhances sensitivity to venetoclax in chronic lymphocytic leukemia.Leukemia. 2017 Oct;31(10):2075-2084. doi: 10.1038/leu.2017.32. Epub 2017 Jan 23. Leukemia. 2017. PMID: 28111464 Free PMC article.
-
BCL-2 Inhibition as Treatment for Chronic Lymphocytic Leukemia.Curr Treat Options Oncol. 2021 Jun 10;22(8):66. doi: 10.1007/s11864-021-00862-z. Curr Treat Options Oncol. 2021. PMID: 34110507 Review.
-
Treatment of Chronic Lymphocytic Leukemia After Discontinuation of Bruton's Tyrosine Kinase Inhibitors.Hematol Oncol Clin North Am. 2021 Aug;35(4):793-806. doi: 10.1016/j.hoc.2021.03.008. Epub 2021 May 27. Hematol Oncol Clin North Am. 2021. PMID: 34174986 Review.
Cited by
-
Cellular Therapy in High-Risk Relapsed/Refractory Chronic Lymphocytic Leukemia and Richter Syndrome.Front Oncol. 2022 Apr 28;12:888109. doi: 10.3389/fonc.2022.888109. eCollection 2022. Front Oncol. 2022. PMID: 35574335 Free PMC article. Review.
-
CD160 receptor in CLL: Current state and future avenues.Front Immunol. 2022 Nov 7;13:1028013. doi: 10.3389/fimmu.2022.1028013. eCollection 2022. Front Immunol. 2022. PMID: 36420268 Free PMC article. Review.
-
The role of allogeneic hematopoietic cell transplantation for chronic lymphocytic leukemia: A review.Front Oncol. 2023 Jan 18;12:1105779. doi: 10.3389/fonc.2022.1105779. eCollection 2022. Front Oncol. 2023. PMID: 36741737 Free PMC article. Review.
-
Outcomes of patients with CLL sequentially resistant to both BCL2 and BTK inhibition.Blood Adv. 2021 Oct 26;5(20):4054-4058. doi: 10.1182/bloodadvances.2021005083. Blood Adv. 2021. PMID: 34478505 Free PMC article.
-
Treatment Refractoriness in Chronic Lymphocytic Leukemia: Old and New Molecular Biomarkers.Int J Mol Sci. 2023 Jun 20;24(12):10374. doi: 10.3390/ijms241210374. Int J Mol Sci. 2023. PMID: 37373521 Free PMC article. Review.
References
-
- Souers AJ, Leverson JD, Boghaert ER, et al. . ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202-208. - PubMed
-
- Stilgenbauer S, Eichhorst B, Schetelig J, et al. . Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17(6):768-778. - PubMed
-
- Kater AP, Seymour JF, Hillmen P, et al. . Fixed duration of venetoclax-rituximab in relapsed/refractory chronic lymphocytic leukemia eradicates minimal residual disease and prolongs survival: post-treatment follow-up of the MURANO phase III study. J Clin Oncol. 2019;37(4):269-277. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

