Aspirin: A Suicide Inhibitor of Carbonic Anhydrase II
- PMID: 32244293
- PMCID: PMC7226357
- DOI: 10.3390/biom10040527
Aspirin: A Suicide Inhibitor of Carbonic Anhydrase II
Abstract
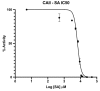
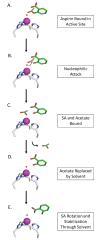
Carbonic anhydrase II (CAII) is a metalloenzyme that catalyzes the reversible hydration/dehydration of CO2/HCO3-. In addition, CAII is attributed to other catalytic reactions, including esterase activity. Aspirin (acetyl-salicylic acid), an everyday over-the-counter drug, has both ester and carboxylic acid moieties. Recently, compounds with a carboxylic acid group have been shown to inhibit CAII. Hence, we hypothesized that Aspirin could act as a substrate for esterase activity, and the product salicylic acid (SA), an inhibitor of CAII. Here, we present the crystal structure of CAII in complex with SA, a product of CAII crystals pre-soaked with Aspirin, to 1.35Å resolution. In addition, we provide kinetic data to support the observation that CAII converts Aspirin to its deacetylated form, SA. This data may also explain the short half-life of Aspirin, with CAII so abundant in blood, and that Aspirin could act as a suicide inhibitor of CAII.
Keywords: Aspirin; X-ray crystallography; carbonic anhydrase; esterase; salicylic acid.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Khalifah R.G. The carbon dioxide hydration activity of carbonic anhydrase I. stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971;246:2561–2573. - PubMed
-
- Lomelino C.L., Andring J.T., McKenna R. Carbonic Anhydrases: Biochemistry, Mechanism of Action and Therapeutic Applications. Nova Science; Hauppauge, NY, USA: 2018. Structural insights into the catalytic mechanism of carbonic anhydrase.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

