Comparative Proteomic Analysis by iTRAQ Reveals that Plastid Pigment Metabolism Contributes to Leaf Color Changes in Tobacco (Nicotiana tabacum) during Curing
- PMID: 32244294
- PMCID: PMC7178154
- DOI: 10.3390/ijms21072394
Comparative Proteomic Analysis by iTRAQ Reveals that Plastid Pigment Metabolism Contributes to Leaf Color Changes in Tobacco (Nicotiana tabacum) during Curing
Abstract
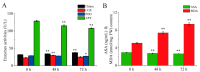
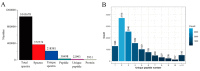
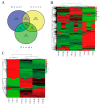
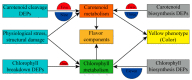
Tobacco (Nicotiana tabacum), is a world's major non-food agricultural crop widely cultivated for its economic value. Among several color change associated biological processes, plastid pigment metabolism is of trivial importance in postharvest plant organs during curing and storage. However, the molecular mechanisms involved in carotenoid and chlorophyll metabolism, as well as color change in tobacco leaves during curing, need further elaboration. Here, proteomic analysis at different curing stages (0 h, 48 h, 72 h) was performed in tobacco cv. Bi'na1 with an aim to investigate the molecular mechanisms of pigment metabolism in tobacco leaves as revealed by the iTRAQ proteomic approach. Our results displayed significant differences in leaf color parameters and ultrastructural fingerprints that indicate an acceleration of chloroplast disintegration and promotion of pigment degradation in tobacco leaves due to curing. In total, 5931 proteins were identified, of which 923 (450 up-regulated, 452 down-regulated, and 21 common) differentially expressed proteins (DEPs) were obtained from tobacco leaves. To elucidate the molecular mechanisms of pigment metabolism and color change, 19 DEPs involved in carotenoid metabolism and 12 DEPs related to chlorophyll metabolism were screened. The results exhibited the complex regulation of DEPs in carotenoid metabolism, a negative regulation in chlorophyll biosynthesis, and a positive regulation in chlorophyll breakdown, which delayed the degradation of xanthophylls and accelerated the breakdown of chlorophylls, promoting the formation of yellow color during curing. Particularly, the up-regulation of the chlorophyllase-1-like isoform X2 was the key protein regulatory mechanism responsible for chlorophyll metabolism and color change. The expression pattern of 8 genes was consistent with the iTRAQ data. These results not only provide new insights into pigment metabolism and color change underlying the postharvest physiological regulatory networks in plants, but also a broader perspective, which prompts us to pay attention to further screen key proteins in tobacco leaves during curing.
Keywords: Nicotiana tabacum; iTRAQ; pigment metabolism; postharvest physiology; ultrastructure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ma C., Liang B., Chang B., Liu L., Yan J., Yang Y., Zhao Z. Transcriptome Profiling Reveals Transcriptional Regulation by DNA Methyltransferase Inhibitor 5-Aza-2′-Deoxycytidine Enhancing Red Pigmentation in Bagged “Granny Smith” Apples (Malus domestica) Int. J. Mol. Sci. 2018;19:3133. doi: 10.3390/ijms19103133. - DOI - PMC - PubMed
-
- Chen J., Funnell K.A., Lewis D.H., Eason J.R., Woolley D.J. Relationship between changes in colour and pigment content during spathe regreening of Zantedeschia ‘Best Gold’. Postharvest Biol. Technol. 2012;67:124–129. doi: 10.1016/j.postharvbio.2011.12.019. - DOI
-
- Yuan Z., Deng L., Yin B., Yao S., Zeng K. Effects of blue LED light irradiation on pigment metabolism of ethephon-degreened mandarin fruit. Postharvest Biol. Technol. 2017;134:45–54. doi: 10.1016/j.postharvbio.2017.08.005. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

