Abnormal Upregulation of GPR17 Receptor Contributes to Oligodendrocyte Dysfunction in SOD1 G93A Mice
- PMID: 32244295
- PMCID: PMC7177925
- DOI: 10.3390/ijms21072395
Abnormal Upregulation of GPR17 Receptor Contributes to Oligodendrocyte Dysfunction in SOD1 G93A Mice
Abstract
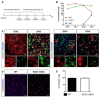
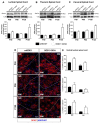
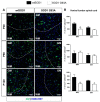
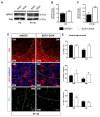
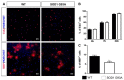
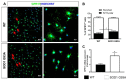
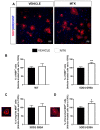
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by progressive loss of motor neurons (MN). Importantly, MN degeneration is intimately linked to oligodendrocyte dysfunction and impaired capacity of oligodendrocyte precursor cells (OPCs) to regenerate the myelin sheath enwrapping and protecting neuronal axons. Thus, improving OPC reparative abilities represents an innovative approach to counteract MN loss. A pivotal regulator of OPC maturation is the P2Y-like G protein-coupled receptor 17 (GPR17), whose role in ALS has never been investigated. In other models of neurodegeneration, an abnormal increase of GPR17 has been invariably associated to myelin defects and its pharmacological manipulation succeeded in restoring endogenous remyelination. Here, we analyzed GPR17 alterations in the SOD1G93A ALS mouse model and assessed in vitro whether this receptor could be targeted to correct oligodendrocyte alterations. Western-blot and immunohistochemical analyses showed that GPR17 protein levels are significantly increased in spinal cord of ALS mice at pre-symptomatic stage; this alteration is exacerbated at late symptomatic phases. Concomitantly, mature oligodendrocytes degenerate and are not successfully replaced. Moreover, OPCs isolated from spinal cord of SOD1G93A mice display defective differentiation compared to control cells, which is rescued by treatment with the GPR17 antagonist montelukast. These data open novel therapeutic perspectives for ALS management.
Keywords: G protein-coupled receptor 17 (GPR17); SOD1G93A ALS mouse model; amyotrophic lateral sclerosis (ALS); montelukast; oligodendrocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Rafałowska J., Dziewulska D. White matter injury in amyotrophic lateral sclerosis (ALS) Folia Neuropathol. 1996;34:87–91. - PubMed
-
- Mollink J., Hiemstra M., Miller K.L., Huszar I.N., Jenkinson M., Raaphorst J., Wiesmann M., Ansorge O., Pallebage-Gamarallage M., van Cappellen van Walsum A.M. White matter changes in the perforant path area in patients with amyotrophic lateral sclerosis. Neuropathol. Appl. Neurobiol. 2019;45:570–585. doi: 10.1111/nan.12555. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

