Involvement of Enteric Glia in Small Intestine Neuromuscular Dysfunction of Toll-Like Receptor 4-Deficient Mice
- PMID: 32244316
- PMCID: PMC7226836
- DOI: 10.3390/cells9040838
Involvement of Enteric Glia in Small Intestine Neuromuscular Dysfunction of Toll-Like Receptor 4-Deficient Mice
Abstract
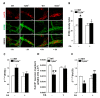
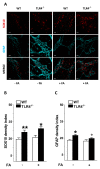
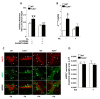
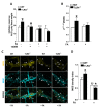
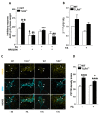
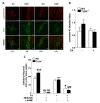
Enteric glial cells (EGCs) influence nitric oxide (NO)- and adenosine diphosphate (ADP)- mediated signaling in the enteric nervous system (ENS). Since Toll-like receptor 4 (TLR4) participates to EGC homoeostasis, this study aimed to evaluate the possible involvement of EGCs in the alterations of the inhibitory neurotransmission in TLR4-/- mice. Ileal segments from male TLR4-/- and wild-type (WT) C57BL/6J mice were incubated with the gliotoxin fluoroacetate (FA). Alterations in ENS morphology and neurochemical coding were investigated by immunohistochemistry whereas neuromuscular responses were determined by recording non-adrenergic non-cholinergic (NANC) relaxations in isometrically suspended isolated ileal preparations. TLR4-/- ileal segments showed increased iNOS immunoreactivity associated with enhanced NANC relaxation, mediated by iNOS-derived NO and sensitive to P2Y1 inhibition. Treatment with FA diminished iNOS immunoreactivity and partially abolished NO- and ADP- mediated relaxation in the TLR4-/- mouse ileum, with no changes of P2Y1 and connexin-43 immunofluorescence distribution in the ENS. After FA treatment, S100β and GFAP immunoreactivity in TLR4-/- myenteric plexus was reduced to levels comparable to those observed in WT. Our findings show the involvement of EGCs in the alterations of ENS architecture and in the increased purinergic and nitrergic-mediated relaxation, determining gut dysmotility in TLR4-/- mice.
Keywords: enteric glial cells; enteric nervous system; fluoroacetate; innate immunity; intestinal motility; knockout mice; small intestine; toll-like receptor 4.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ochoa-Cortes F., Turco F., Linan-Rico A., Soghomonyan S., Whitaker E., Wehner S., Cuomo R., Christofi F.L. Enteric Glial Cells: A New Frontier in Neurogastroenterology and Clinical Target for Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2016;22:433–449. doi: 10.1097/MIB.0000000000000667. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

