Recent Advances in the Synthesis of Oxazole-Based Molecules via van Leusen Oxazole Synthesis
- PMID: 32244317
- PMCID: PMC7180750
- DOI: 10.3390/molecules25071594
Recent Advances in the Synthesis of Oxazole-Based Molecules via van Leusen Oxazole Synthesis
Abstract
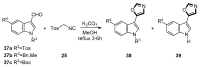
Oxazole compounds, including one nitrogen atom and one oxygen atom in a five-membered heterocyclic ring, are present in various biological activities. Due to binding with a widespread spectrum of receptors and enzymes easily in biological systems through various non-covalent interactions, oxazole-based molecules are becoming a kind of significant heterocyclic nucleus, which have received attention from researchers globally, leading them to synthesize diverse oxazole derivatives. The van Leusen reaction, based on tosylmethylisocyanides (TosMICs), is one of the most appropriate strategies to prepare oxazole-based medicinal compounds. In this review, we summarize the recent advances of the synthesis of oxazole-containing molecules utilizing the van Leusen oxazole synthesis from 1972, aiming to look for potential oxazole-based medicinal compounds, which are valuable information for drug discovery and synthesis.
Keywords: TosMICs; oxazole; synthesis; van Leusen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

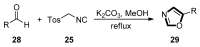




















Similar articles
-
Synthesis of Imidazole-Based Medicinal Molecules Utilizing the van Leusen Imidazole Synthesis.Pharmaceuticals (Basel). 2020 Mar 3;13(3):37. doi: 10.3390/ph13030037. Pharmaceuticals (Basel). 2020. PMID: 32138202 Free PMC article. Review.
-
Recent advance in oxazole-based medicinal chemistry.Eur J Med Chem. 2018 Jan 20;144:444-492. doi: 10.1016/j.ejmech.2017.12.044. Epub 2017 Dec 14. Eur J Med Chem. 2018. PMID: 29288945 Review.
-
Natural and synthetic 5-(3'-indolyl)oxazoles: Biological activity, chemical synthesis and advanced molecules.Med Res Rev. 2025 Jan;45(1):97-143. doi: 10.1002/med.22078. Epub 2024 Aug 16. Med Res Rev. 2025. PMID: 39152525 Review.
-
Therapeutic potential of oxazole scaffold: a patent review (2006-2017).Expert Opin Ther Pat. 2018 Nov;28(11):783-812. doi: 10.1080/13543776.2018.1526280. Epub 2018 Sep 26. Expert Opin Ther Pat. 2018. PMID: 30239247 Review.
-
Oxazole-Based Compounds As Anticancer Agents.Curr Med Chem. 2019;26(41):7337-7371. doi: 10.2174/0929867326666181203130402. Curr Med Chem. 2019. PMID: 30501590 Review.
Cited by
-
Dissimilarity in the Chemical Behavior of Osmaoxazolium Salts and Osmaoxazoles: Two Different Aromatic Metalladiheterocycles.Organometallics. 2021 Dec 27;40(24):4150-4162. doi: 10.1021/acs.organomet.1c00621. Epub 2021 Dec 14. Organometallics. 2021. PMID: 35264819 Free PMC article.
-
Iodonium Cation-Pool Electrolysis for the Three-Component Synthesis of 1,3-Oxazoles.Chemistry. 2021 Jan 7;27(2):605-608. doi: 10.1002/chem.202004140. Epub 2020 Dec 3. Chemistry. 2021. PMID: 33270278 Free PMC article.
-
Pharmacological investigations of newly synthesized oxazolones and imidazolones as COX-2 inhibitors.Saudi Pharm J. 2024 Nov;32(11):102191. doi: 10.1016/j.jsps.2024.102191. Epub 2024 Oct 19. Saudi Pharm J. 2024. PMID: 39507051 Free PMC article.
-
2-Amino-4-aryl-5-oxo-4,5-dihydropyrano[3,2-c]chromene-3-carbonitriles with Microtubule-Disruptive, Centrosome-Declustering, and Antiangiogenic Effects in vitro and in vivo.ChemMedChem. 2022 May 18;17(10):e202200064. doi: 10.1002/cmdc.202200064. Epub 2022 Mar 16. ChemMedChem. 2022. PMID: 35226402 Free PMC article.
-
A New Naphthopyran Derivative Combines c-Myb Inhibition, Microtubule-Targeting Effects, and Antiangiogenic Properties.ACS Med Chem Lett. 2022 Oct 31;13(11):1783-1790. doi: 10.1021/acsmedchemlett.2c00403. eCollection 2022 Nov 10. ACS Med Chem Lett. 2022. PMID: 36385941 Free PMC article.
References
-
- Aljaar N., Gujjarappa R., Al-Refai M., Shtaiwi M., Malakar C.C. Overview on recent approaches towards synthesis of 2-keto-annulated oxazole derivatives. J. Heterocycl. Chem. 2019;56:2730–2743. doi: 10.1002/jhet.3673. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

