Corticotropin-Releasing Factor Family: A Stress Hormone-Receptor System's Emerging Role in Mediating Sex-Specific Signaling
- PMID: 32244319
- PMCID: PMC7226788
- DOI: 10.3390/cells9040839
Corticotropin-Releasing Factor Family: A Stress Hormone-Receptor System's Emerging Role in Mediating Sex-Specific Signaling
Abstract
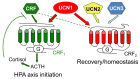
No organ in the body is impervious to the effects of stress, and a coordinated response from all organs is essential to deal with stressors. A dysregulated stress response that fails to bring systems back to homeostasis leads to compromised function and ultimately a diseased state. The components of the corticotropin-releasing factor (CRF) family, an ancient and evolutionarily conserved stress hormone-receptor system, helps both initiate stress responses and bring systems back to homeostasis once the stressors are removed. The mammalian CRF family comprises of four known agonists, CRF and urocortins (UCN1-3), and two known G protein-coupled receptors (GPCRs), CRF1 and CRF2. Evolutionarily, precursors of CRF- and urocortin-like peptides and their receptors were involved in osmoregulation/diuretic functions, in addition to nutrient sensing. Both CRF and UCN1 peptide hormones as well as their receptors appeared after a duplication event nearly 400 million years ago. All four agonists and both CRF receptors show sex-specific changes in expression and/or function, and single nucleotide polymorphisms are associated with a plethora of human diseases. CRF receptors harbor N-terminal cleavable peptide sequences, conferring biased ligand properties. CRF receptors have the ability to heteromerize with each other as well as with other GPCRs. Taken together, CRF receptors and their agonists due to their versatile functional adaptability mediate nuanced responses and are uniquely positioned to orchestrate sex-specific signaling and function in several tissues.
Keywords: BNST; CRF; GPCR; cardiovascular; diabetes; gut; inflammatory bowel disease; pancreas; reproduction; sexually dimorphic; urocortins.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures