A Syringe-Based Biosensor to Rapidly Detect Low Levels of Escherichia Coli (ECOR13) in Drinking Water Using Engineered Bacteriophages
- PMID: 32244369
- PMCID: PMC7181147
- DOI: 10.3390/s20071953
A Syringe-Based Biosensor to Rapidly Detect Low Levels of Escherichia Coli (ECOR13) in Drinking Water Using Engineered Bacteriophages
Abstract
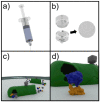
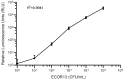
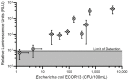
A sanitized drinking water supply is an unconditional requirement for public health and the overall prosperity of humanity. Potential microbial and chemical contaminants of drinking water have been identified by a joint effort between the World Health Organization (WHO) and the United Nations Children's Fund (UNICEF), who together establish guidelines that define, in part, that the presence of Escherichia coli (E. coli) in drinking water is an indication of inadequate sanitation and a significant health risk. As E. coli is a nearly ubiquitous resident of mammalian gastrointestinal tracts, no detectable counts in 100 mL of drinking water is the standard used worldwide as an indicator of sanitation. The currently accepted EPA method relies on filtration, followed by growth on selective media, and requires 24-48 h from sample to results. In response, we developed a rapid bacteriophage-based detection assay with detection limit capabilities comparable to traditional methods in less than a quarter of the time. We coupled membrane filtration with selective enrichment using genetically engineered bacteriophages to identify less than 20 colony forming units (CFU) E. coli in 100 mL drinking water within 5 h. The combination of membrane filtration with phage infection produced a novel assay that demonstrated a rapid, selective, and sensitive detection of an indicator organism in large volumes of drinking water as recommended by the leading world regulatory authorities.
Keywords: E.coli; bacteriophage; drinking water; rapid detection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Filter-based assay for Escherichia coli in aqueous samples using bacteriophage-based amplification.Anal Chem. 2013 Aug 6;85(15):7213-20. doi: 10.1021/ac400961b. Epub 2013 Jul 12. Anal Chem. 2013. PMID: 23848541
-
A phage-based assay for the rapid, quantitative, and single CFU visualization of E. coli (ECOR #13) in drinking water.Sci Rep. 2018 Oct 2;8(1):14630. doi: 10.1038/s41598-018-33097-4. Sci Rep. 2018. PMID: 30279488 Free PMC article.
-
Reporter bacteriophage T7NLC utilizes a novel NanoLuc::CBM fusion for the ultrasensitive detection of Escherichia coli in water.Analyst. 2018 Aug 20;143(17):4074-4082. doi: 10.1039/c8an00781k. Analyst. 2018. PMID: 30069563
-
Escherichia coli: the best biological drinking water indicator for public health protection.Symp Ser Soc Appl Microbiol. 2000;(29):106S-116S. doi: 10.1111/j.1365-2672.2000.tb05338.x. Symp Ser Soc Appl Microbiol. 2000. PMID: 10880185 Review.
-
Detection and enumeration of coliforms in drinking water: current methods and emerging approaches.J Microbiol Methods. 2002 Mar;49(1):31-54. doi: 10.1016/s0167-7012(01)00351-7. J Microbiol Methods. 2002. PMID: 11777581 Review.
Cited by
-
Luminescent Phage-Based Detection of Klebsiella pneumoniae: From Engineering to Diagnostics.Pharmaceuticals (Basel). 2021 Apr 9;14(4):347. doi: 10.3390/ph14040347. Pharmaceuticals (Basel). 2021. PMID: 33918942 Free PMC article.
-
Recent Progress in the Detection of Bacteria Using Bacteriophages: A Review.Viruses. 2020 Aug 3;12(8):845. doi: 10.3390/v12080845. Viruses. 2020. PMID: 32756438 Free PMC article. Review.
-
Biosensor: An Emerging Technological Tool for Microorganisms and Its Disease Diagnosis.Indian J Microbiol. 2023 Dec;63(4):395-397. doi: 10.1007/s12088-023-01142-0. Epub 2023 Nov 17. Indian J Microbiol. 2023. PMID: 38031605 Free PMC article. No abstract available.
-
Application of Bacteriophages in Nanotechnology.Nanomaterials (Basel). 2020 Sep 29;10(10):1944. doi: 10.3390/nano10101944. Nanomaterials (Basel). 2020. PMID: 33003494 Free PMC article. Review.
-
The Use of Bacteriophages in Biotechnology and Recent Insights into Proteomics.Antibiotics (Basel). 2022 May 13;11(5):653. doi: 10.3390/antibiotics11050653. Antibiotics (Basel). 2022. PMID: 35625297 Free PMC article. Review.
References
-
- Assembly U.N.G. The human right to water and sanitation. UN Resolut. 2010;64:292.
-
- Ashbolt N., Fujioka R., Glymph T., McGee C., Schaub S., Sobsey M., Toranzos G. Report of the Experts Scientific Workshop on Critical Research Needs for the Development of New or Revised Recreational Water Quality Criteria. EPA; Wasington, DC, USA: 2007. Pathogen indicators, and indicators of fecal contamination; pp. 35–56.
-
- De Vrese M., Schrezenmeir J. Probiotics, Prebiotics, and Synbiotics. In: Stahl U., Donalies U.E., Nevoigt E., editors. Food Biotechnology. Advances in Biochemical Engineering/Biotechnology. Volume 111 Springer; Berlin, Heidelberg: 2008. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

