Antiproliferative and Antimigration Activities of Fluoro-Neplanocin A via Inhibition of Histone H3 Methylation in Triple-Negative Breast Cancer
- PMID: 32244385
- PMCID: PMC7226301
- DOI: 10.3390/biom10040530
Antiproliferative and Antimigration Activities of Fluoro-Neplanocin A via Inhibition of Histone H3 Methylation in Triple-Negative Breast Cancer
Abstract
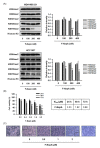
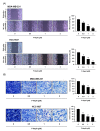
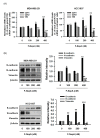
Triple-negative breast cancer (TNBC) is among the most aggressive and potentially metastatic malignancies. Most affected patients have poor clinical outcomes due to the lack of specific molecular targets on tumor cells. The upregulated expression of disruptor of telomeric silencing 1-like (DOT1L), a histone methyltransferase specific for the histone H3 lysine 79 residue (H3K79), is strongly correlated with TNBC cell aggressiveness. Therefore, DOT1L is considered a potential molecular target in TNBC. Fluoro-neplanocin A (F-NepA), an inhibitor of S-adenosylhomocysteine hydrolase, exhibited potent antiproliferative activity against various types of cancer cells, including breast cancers. However, the molecular mechanism underlying the anticancer activity of F-NepA in TNBC cells remains to be elucidated. We determined that F-NepA exhibited a higher growth-inhibitory activity against TNBC cells relative to non-TNBC breast cancer and normal breast epithelial cells. Moreover, F-NepA effectively downregulated the level of H3K79me2 in MDA-MB-231 TNBC cells by inhibiting DOT1L activity. F-NepA also significantly inhibited TNBC cell migration and invasion. These activities of F-NepA might be associated with the upregulation of E-cadherin and downregulation of N-cadherin and Vimentin in TNBC cells. Taken together, these data highlight F-NepA as a strong potential candidate for the targeted treatment of high-DOT1L-expressing TNBC.
Keywords: DOT1L; H3K79me2; fluoro-neplanocin A; histone H3 lysine methylation; metastasis; triple-negative breast cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Byun W.S., Kim W.K., Han H.J., Chung H.-J., Jang K., Kim H.S., Kim S., Kim D., Bae E.S., Park S., et al. Targeting histone methyltransferase DOT1L by a novel psammaplin A analog inhibits growth and metastasis of triple-negative breast cancer. Mol. Ther. Oncolytics. 2019;15:140–152. doi: 10.1016/j.omto.2019.09.005. - DOI - PMC - PubMed
-
- Colleoni M., Sun Z., Price K.N., Karlsson P., Forbes J.F., Thürlimann B., Gianni L., Castiglione M., Gelber R.D., Coates A.S., et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: Results from the international breast cancer study group trials I to V. J. Clin. Oncol. 2016;34:927–935. doi: 10.1200/JCO.2015.62.3504. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

