Role of PA2G4P4 pseudogene in bladder cancer tumorigenesis
- PMID: 32244410
- PMCID: PMC7235711
- DOI: 10.3390/biology9040066
Role of PA2G4P4 pseudogene in bladder cancer tumorigenesis
Abstract
Background: Many pseudogenes possess biological activities and play important roles in the pathogenesis of various types of cancer including bladder cancer (BlCa), which still lacks suitable molecular biomarkers. Recently, pseudogenes were found to be significantly enriched in a pan-cancer classification based on the Cancer Genome Atlas gene expression data. Among them, the top-ranking pseudogene was the proliferation-associated 2G4 pseudogene 4 (PA2G4P4).
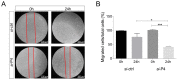
Methods: Genomic and transcript features of PA2G4P4 were determined by GeneBank database analysis followed by 5' RACE experiments. Therefore, we conducted a retrospective molecular study on a cohort of 45 patients of BlCa. PA2G4P4 expression was measured by RT-qPCR, whereas PA2G4P4 transcript distribution was analyzed by in situ hybridization on both normal and cancerous histological sections and compared to the immunolocalization of its parental PA2G4/EBP1 protein. Finally, we tested the effects of PA2G4P4 depletion on proliferation, migration, and death of BlCa cells.
Results: We showed for the first time PA2G4P4 overexpression in BlCa tissues and in cell lines. PA2G4P4 distribution strictly overlaps PA2G4/EBP1 protein localization. Moreover, we showed that PA2G4P4 knockdown affects both proliferation and migration of BlCa cells, highlighting its potential oncogenic role.
Conclusions: PA2G4P4 may play a functional role as an oncogene in BlCa development, suggesting it as a good candidate for future investigation and new clinical applications.
Keywords: EBP1; bladder cancer; expression; noncoding RNA; oncogene.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

