Partners in Crime: The Interplay of Proteins and Membranes in Regulated Necrosis
- PMID: 32244433
- PMCID: PMC7177786
- DOI: 10.3390/ijms21072412
Partners in Crime: The Interplay of Proteins and Membranes in Regulated Necrosis
Abstract
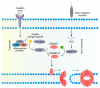
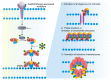
Pyroptosis, necroptosis, and ferroptosis are well-characterized forms of regulated necrosis that have been associated with human diseases. During regulated necrosis, plasma membrane damage facilitates the movement of ions and molecules across the bilayer, which finally leads to cell lysis and release of intracellular content. Therefore, these types of cell death have an inflammatory phenotype. Each type of regulated necrosis is mediated by a defined machinery comprising protein and lipid molecules. Here, we discuss how the interaction and reshaping of these cellular components are essential and distinctive processes during pyroptosis, necroptosis, and ferroptosis. We point out that although the plasma membrane is the common target in regulated necrosis, different mechanisms of permeabilization have emerged depending on the cell death form. Pore formation by gasdermins (GSDMs) is a hallmark of pyroptosis, while mixed lineage kinase domain-like (MLKL) protein facilitates membrane permeabilization in necroptosis, and phospholipid peroxidation leads to membrane damage in ferroptosis. This diverse repertoire of mechanisms leading to membrane permeabilization contributes to define the specific inflammatory and immunological outcome of each type of regulated necrosis. Current efforts are focused on new therapies that target critical protein and lipid molecules on these pathways to fight human pathologies associated with inflammation.
Keywords: inflammation; membrane permeabilization; pores; protein-lipid interactions; regulated necrosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Galluzzi L., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Agostinis P., Alnemri E.S., Altucci L., Amelio I., Andrews D.W., et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature committee on cell death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. - DOI - PMC - PubMed
-
- Espiritu R.A., Pedrera L., Ros U. Tuning the way to die: Implications of membrane perturbations in necroptosis. In: Iglič A., Garcia-Sáez A., Rappolt M., editors. Advances in Biomembranes and Lipid Self-Assembly. Academic Press; Cambridge, MA, USA: 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

