Chronic Regulation of miR-124-3p in the Perilesional Cortex after Experimental and Human TBI
- PMID: 32244461
- PMCID: PMC7177327
- DOI: 10.3390/ijms21072418
Chronic Regulation of miR-124-3p in the Perilesional Cortex after Experimental and Human TBI
Abstract
Traumatic brain injury (TBI) dysregulates microRNAs, which are the master regulators of gene expression. Here we investigated the changes in a brain-enriched miR-124-3p, which is known to associate with major post-injury pathologies, such as neuroinflammation. RT-qPCR of the rat tissue sampled at 7 d and 3 months in the perilesional cortex adjacent to the necrotic lesion core (aPeCx) revealed downregulation of miR-124-3p at 7 d (fold-change (FC) 0.13, p < 0.05 compared with control) and 3 months (FC 0.40, p < 0.05) post-TBI. In situ hybridization confirmed the downregulation of miR-124-3p at 7 d and 3 months post-TBI in the aPeCx (both p < 0.01). RT-qPCR confirmed the upregulation of the miR-124-3p target Stat3 in the aPeCx at 7 d post-TBI (7-fold, p < 0.05). mRNA-Seq revealed 312 downregulated and 311 upregulated miR-124 targets (p < 0.05). To investigate whether experimental findings translated to humans, we performed in situ hybridization of miR-124-3p in temporal lobe autopsy samples of TBI patients. Our data revealed downregulation of miR-124-3p in individual neurons of cortical layer III. These findings indicate a persistent downregulation of miR-124-3p in the perilesional cortex that might contribute to post-injury neurodegeneration and inflammation.
Keywords: bioinformatics; biomarker; epileptogenesis; miR-124-3p; microRNA; traumatic brain injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
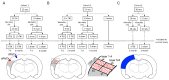
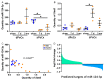
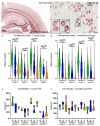

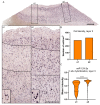
Similar articles
-
Co-Expression Network Analysis of MicroRNAs and Proteins in Severe Traumatic Brain Injury: A Systematic Review.Cells. 2021 Sep 14;10(9):2425. doi: 10.3390/cells10092425. Cells. 2021. PMID: 34572074 Free PMC article.
-
miR-124-3p is a chronic regulator of gene expression after brain injury.Cell Mol Life Sci. 2018 Dec;75(24):4557-4581. doi: 10.1007/s00018-018-2911-z. Epub 2018 Aug 28. Cell Mol Life Sci. 2018. PMID: 30155647 Free PMC article.
-
Elevated Acute Plasma miR-124-3p Level Relates to Evolution of Larger Cortical Lesion Area after Traumatic Brain Injury.Neuroscience. 2020 May 1;433:21-35. doi: 10.1016/j.neuroscience.2020.02.045. Epub 2020 Mar 4. Neuroscience. 2020. PMID: 32142864
-
Discovery and Validation of Circulating microRNAs as Biomarkers for Epileptogenesis after Experimental Traumatic Brain Injury-The EPITARGET Cohort.Int J Mol Sci. 2023 Feb 1;24(3):2823. doi: 10.3390/ijms24032823. Int J Mol Sci. 2023. PMID: 36769143 Free PMC article.
-
MicroRNA-21 in the Pathogenesis of Traumatic Brain Injury.Neurochem Res. 2018 Oct;43(10):1863-1868. doi: 10.1007/s11064-018-2602-z. Epub 2018 Jul 31. Neurochem Res. 2018. PMID: 30066160 Review.
Cited by
-
Inhibition of long non-coding RNA HOXA11-AS against neuroinflammation in Parkinson's disease model via targeting miR-124-3p mediated FSTL1/NF-κB axis.Aging (Albany NY). 2021 Apr 4;13(8):11455-11469. doi: 10.18632/aging.202837. Epub 2021 Apr 4. Aging (Albany NY). 2021. PMID: 33839699 Free PMC article.
-
Evaluation of a Set of miRNAs in 26 Cases of Fatal Traumatic Brain Injuries.Int J Mol Sci. 2023 Jun 29;24(13):10836. doi: 10.3390/ijms241310836. Int J Mol Sci. 2023. PMID: 37446013 Free PMC article.
-
Aquaporin‑1 regulates microglial polarization and inflammatory response in traumatic brain injury.Int J Mol Med. 2025 Mar;55(3):41. doi: 10.3892/ijmm.2025.5482. Epub 2025 Jan 3. Int J Mol Med. 2025. PMID: 39749692 Free PMC article.
-
Co-Expression Network Analysis of MicroRNAs and Proteins in Severe Traumatic Brain Injury: A Systematic Review.Cells. 2021 Sep 14;10(9):2425. doi: 10.3390/cells10092425. Cells. 2021. PMID: 34572074 Free PMC article.
-
Characterization of Exosomes and Exosomal RNAs Isolated from Post-Mortem Body Fluids for Molecular Forensic Diagnosis.Diagnostics (Basel). 2022 Sep 5;12(9):2153. doi: 10.3390/diagnostics12092153. Diagnostics (Basel). 2022. PMID: 36140554 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

