Targeting PKCθ Promotes Satellite Cell Self-Renewal
- PMID: 32244482
- PMCID: PMC7177808
- DOI: 10.3390/ijms21072419
Targeting PKCθ Promotes Satellite Cell Self-Renewal
Abstract
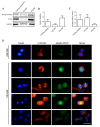
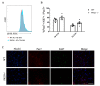
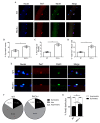
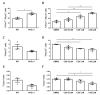
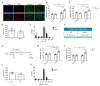
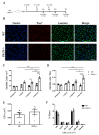
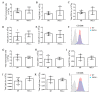
Skeletal muscle regeneration following injury depends on the ability of satellite cells (SCs) to proliferate, self-renew, and eventually differentiate. The factors that regulate the process of self-renewal are poorly understood. In this study we examined the role of PKCθ in SC self-renewal and differentiation. We show that PKCθ is expressed in SCs, and its active form is localized to the chromosomes, centrosomes, and midbody during mitosis. Lack of PKCθ promotes SC symmetric self-renewal division by regulating Pard3 polarity protein localization, without affecting the overall proliferation rate. Genetic ablation of PKCθ or its pharmacological inhibition in vivo did not affect SC number in healthy muscle. By contrast, after induction of muscle injury, lack or inhibition of PKCθ resulted in a significant expansion of the quiescent SC pool. Finally, we show that lack of PKCθ does not alter the inflammatory milieu after acute injury in muscle, suggesting that the enhanced self-renewal ability of SCs in PKCθ-/- mice is not due to an alteration in the inflammatory milieu. Together, these results suggest that PKCθ plays an important role in SC self-renewal by stimulating their expansion through symmetric division, and it may represent a promising target to manipulate satellite cell self-renewal in pathological conditions.
Keywords: Protein kinase C θ; muscle regeneration; satellite cells; self-renewal.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

