Use of Pyrazole Hydrogen Bonding in Tripodal Complexes to Form Self Assembled Homochiral Dimers
- PMID: 32244503
- PMCID: PMC7178049
- DOI: 10.3390/ma13071595
Use of Pyrazole Hydrogen Bonding in Tripodal Complexes to Form Self Assembled Homochiral Dimers
Abstract
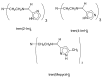
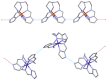
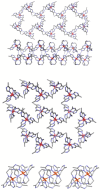
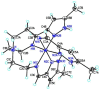
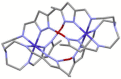
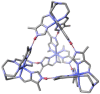
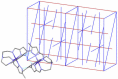
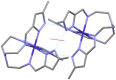
The 3:1 condensation of 5-methyl-1H-pyrazole-3-carboxaldehyde (MepyrzH) with tris(2-aminoethyl)amine (tren) gives the tripodal ligand tren(MePyrzH)3. Aerial oxidation of a solution of cobalt(II) with this ligand in the presence of base results in the isolation of the insoluble Co(tren)(MePyrz)3. This complex reacts with acids, HCl/NaClO4, NH4ClO4, NH4BF4, and NH4I to give the crystalline compounds Co(tren)(MePyrzH)3(ClO4)3, {[Co(tren)(MePyrzH0.5)3](ClO4)1.5}2 {[Co(tren)(MePyrzH0.5)3](BF4)1.5}2 and [Co(tren)(MePyrzH)3][Co(tren)(MePyrzH)3]I2. The latter three complexes are dimeric, held together by three Npyrazole -H…Npyrazolate hydrogen bonds. The structures and symmetries of these homochiral dimers or pseudodimers are discussed in terms of their space group. Possible applications of these complexes by incorporation into new materials are mentioned.
Keywords: cobalt; crystal structure; dimer; hydrogen bonding; pyrazole; supramolecular.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Chiral recognition and conglomerate crystallization induced by self-organization of cobalt(III) complexes of a tripodal ligand containing three imidazole groups.Inorg Chem. 2007 Oct 1;46(20):8170-81. doi: 10.1021/ic070286+. Epub 2007 Sep 7. Inorg Chem. 2007. PMID: 17824602
-
Synthesis of homochiral tris(2-alkyl-2-aminoethyl)amine derivatives from chiral alpha-amino aldehydes and their application in the synthesis of water soluble chelators.Inorg Chem. 2001 Jun 18;40(13):3208-16. doi: 10.1021/ic001021x. Inorg Chem. 2001. PMID: 11399194
-
Novel copper(II) induced formation of a porphyrinogen derivative: X-ray structural, spectroscopic, and electrochemical studies of porphyrinogen complexes of Cu(II) and Co(III) complex of a trispyrazolyl tripodal ligand.Inorg Chem. 2002 Nov 4;41(22):5803-9. doi: 10.1021/ic0112185. Inorg Chem. 2002. PMID: 12401086
-
The encapsulation of ferrocyanide by copper(II) complexes of tripodal tetradentate ligands. Novel H-bonding networks incorporating heptanuclear and pentanuclear heterometallic assemblies.Inorg Chem. 2001 Aug 27;40(18):4696-704. doi: 10.1021/ic001452f. Inorg Chem. 2001. PMID: 11511218
-
Synthesis, characterization, and structures of copper(II)-thiosulfate complexes incorporating tripodal tetraamine ligands.Inorg Chem. 2004 Oct 18;43(21):6568-78. doi: 10.1021/ic0492800. Inorg Chem. 2004. PMID: 15476353
Cited by
-
Discovery of ICOS-targeted small molecules using affinity selection mass spectrometry screening.bioRxiv [Preprint]. 2024 Aug 7:2024.08.04.606538. doi: 10.1101/2024.08.04.606538. bioRxiv. 2024. Update in: ChemMedChem. 2024 Nov 18;19(22):e202400545. doi: 10.1002/cmdc.202400545. PMID: 39149231 Free PMC article. Updated. Preprint.
-
catena-Poly[[tetra-kis-(3,5-dimethyl-1H-pyrazole-κN 2)copper(II)]-μ2-sulfato-κ2 O:O']: crystal structure and Hirshfeld surface analysis of a CuII coordination polymer.Acta Crystallogr E Crystallogr Commun. 2022 Mar 24;78(Pt 4):433-438. doi: 10.1107/S2056989022002894. eCollection 2022 Apr 1. Acta Crystallogr E Crystallogr Commun. 2022. PMID: 35492287 Free PMC article.
-
Discovery of ICOS-Targeted Small Molecules Using Affinity Selection Mass Spectrometry Screening.ChemMedChem. 2024 Nov 18;19(22):e202400545. doi: 10.1002/cmdc.202400545. Epub 2024 Oct 22. ChemMedChem. 2024. PMID: 39269728
-
Mechanistic Investigations into Amination of Unactivated Arenes via Cation Radical Accelerated Nucleophilic Aromatic Substitution.J Am Chem Soc. 2022 Aug 24;144(33):15118-15131. doi: 10.1021/jacs.2c04577. Epub 2022 Aug 9. J Am Chem Soc. 2022. PMID: 35944280 Free PMC article.
References
-
- Ikuta Y., Ooidemizu M., Yamahata Y., Yamada M., Osa S., Matsumoto N., Iijima S., Sunatsuki Y., Kojima M., Dahan F., et al. A New Family of Spin Crossover Complexes with a Tripod Ligand Containing Three Imidazoles: Synthesis, Characterization, and Magnetic Properties of [FeIIH3LMe](NO3)2·1.5H2O, [FeIIILMe]·3.5H2O, [FeIIH3LMe][FeIILMe]NO3, and [FeIIH3LMe][FeIIILMe](NO3)2 (H3LMe = Tris[2-(((2-methylimidazol-4-yl)methylidene)amino)ethyl]amine) Inorg. Chem. 2003;42:7001–7017. - PubMed
-
- Yamada M., Ooidemizu M., Ikuta Y., Osa S., Matsumoto N., Iijima S., Kojima M., Dahan F., Tuchagues J.P. Interlayer Interaction of Two-Dimensional Layered Spin Crossover Complexes [FeIIH3LMe][FeIILMe]X (X− = ClO4−, BF4−, PF6−, AsF6−, and SbF6−; H3LMe = Tris[2-(((2-methylimidazol-4-yl)methylidene)amino)ethyl]amine) Inorg. Chem. 2003;42:8406. doi: 10.1021/ic034439e. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

