Palstimolide A: A Complex Polyhydroxy Macrolide with Antiparasitic Activity
- PMID: 32244512
- PMCID: PMC7180531
- DOI: 10.3390/molecules25071604
Palstimolide A: A Complex Polyhydroxy Macrolide with Antiparasitic Activity
Abstract
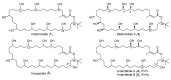
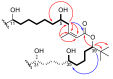
Marine Cyanobacteria (blue-green algae) have been shown to possess an enormous potential to produce structurally diverse natural products that exhibit a broad spectrum of potent biological activities, including cytotoxic, antifungal, antiparasitic, antiviral, and antibacterial activities. Here, we report the isolation and structure determination of palstimolide A, a complex polyhydroxy macrolide with a 40-membered ring that was isolated from a tropical marine cyanobacterium collected at Palmyra Atoll. NMR-guided fractionation in combination with MS2-based molecular networking and isolation via HPLC yielded 0.7 mg of the pure compound. The small quantity isolated along with the presence of significant signal degeneracy in both the 1H and 13C-NMR spectra complicated the structure elucidation of palstimolide A. Various NMR experiments and solvent systems were employed, including the LRHSQMBC experiment that allows the detection of long-range 1H-13C correlation data across 4-, 5-, and even 6-bonds. This expanded NMR data set enabled the elucidation of the palstimolide's planar structure, which is characterized by several 1,5-disposed hydroxy groups as well as a tert-butyl group. The compound showed potent antimalarial activity with an IC50 of 223 nM as well as interesting anti-leishmanial activity with an IC50 of 4.67 µM.
Keywords: antimalarial agents; cyanobacteria; leishmaniosis; macrolide; malaria; natural products.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Burja A.M., Banaigs B., Abou-Mansour E., Grant Burgess J., Wright P.C. Marine cyanobacteria—A prolific source of natural products. Tetrahedron. 2001;57:9347–9377. doi: 10.1016/S0040-4020(01)00931-0. - DOI
-
- Tan L.T. Filamentous tropical marine cyanobacteria: A rich source of natural products for anticancer drug discovery. J. Appl. Phycol. 2010;22:659–676. doi: 10.1007/s10811-010-9506-x. - DOI

