CBD Reverts the Mesenchymal Invasive Phenotype of Breast Cancer Cells Induced by the Inflammatory Cytokine IL-1β
- PMID: 32244518
- PMCID: PMC7177247
- DOI: 10.3390/ijms21072429
CBD Reverts the Mesenchymal Invasive Phenotype of Breast Cancer Cells Induced by the Inflammatory Cytokine IL-1β
Abstract
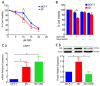
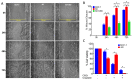
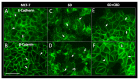
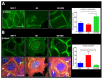
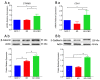
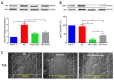
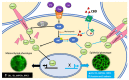
Cannabidiol (CBD) has been used to treat a variety of cancers and inflammatory conditions with controversial results. In previous work, we have shown that breast cancer MCF-7 cells, selected by their response to inflammatory IL-1β cytokine, acquire a malignant phenotype (6D cells) through an epithelial-mesenchymal transition (EMT). We evaluated CBD as a potential inhibitor of this transition and inducer of reversion to a non-invasive phenotype. It decreased 6D cell viability, downregulating expression of receptor CB1. The CBD blocked migration and progression of the IL-1β-induced signaling pathway IL-1β/IL-1RI/β-catenin, the driver of EMT. Cannabidiol reestablished the epithelial organization lost by dispersion of the cells and re-localized E-cadherin and β-catenin at the adherens junctions. It also prevented β-catenin nuclear translocation and decreased over-expression of genes for ∆Np63α, BIRC3, and ID1 proteins, induced by IL-1β for acquisition of malignant features. Cannabidiol inhibited the protein kinase B (AKT) activation, a crucial effector in the IL-1β/IL-1RI/β-catenin pathway, indicating that at this point there is crosstalk between IL-1β and CBD signaling which results in phenotype reversion. Our 6D cell system allowed step-by-step analysis of the phenotype transition and better understanding of mechanisms by which CBD blocks and reverts the effects of inflammatory IL-1β in the EMT.
Keywords: CBD; cancer treatment; inflammatory IL1β; phenotype reversion; signaling pathways.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Proteomic Analysis of Invasive Breast Cancer Cells Treated with CBD Reveals Proteins Associated with the Reversal of Their Epithelial-Mesenchymal Transition Induced by IL-1β.Int J Mol Sci. 2025 May 15;26(10):4721. doi: 10.3390/ijms26104721. Int J Mol Sci. 2025. PMID: 40429863 Free PMC article.
-
A novel β-catenin signaling pathway activated by IL-1β leads to the onset of epithelial-mesenchymal transition in breast cancer cells.Cancer Lett. 2014 Nov 1;354(1):164-71. doi: 10.1016/j.canlet.2014.08.015. Epub 2014 Aug 15. Cancer Lett. 2014. PMID: 25135221
-
IL-1β Inflammatory Cytokine-Induced TP63 Isoform ∆NP63α Signaling Cascade Contributes to Cisplatin Resistance in Human Breast Cancer Cells.Int J Mol Sci. 2019 Jan 11;20(2):270. doi: 10.3390/ijms20020270. Int J Mol Sci. 2019. PMID: 30641908 Free PMC article.
-
Exploring the Crosstalk between Inflammation and Epithelial-Mesenchymal Transition in Cancer.Mediators Inflamm. 2021 Jun 14;2021:9918379. doi: 10.1155/2021/9918379. eCollection 2021. Mediators Inflamm. 2021. PMID: 34220337 Free PMC article. Review.
-
Biological effects of Cannabidiol on normal human healthy cell populations: Systematic review of the literature.Biomed Pharmacother. 2020 Dec;132:110728. doi: 10.1016/j.biopha.2020.110728. Epub 2020 Oct 7. Biomed Pharmacother. 2020. PMID: 33038581
Cited by
-
Antimicrobial and Cytotoxic Effects of Cannabinoids: An Updated Review with Future Perspectives and Current Challenges.Pharmaceuticals (Basel). 2022 Oct 6;15(10):1228. doi: 10.3390/ph15101228. Pharmaceuticals (Basel). 2022. PMID: 36297340 Free PMC article. Review.
-
The evolution of cannabinoid receptors in cancer.WIREs Mech Dis. 2023 Jul-Aug;15(4):e1602. doi: 10.1002/wsbm.1602. Epub 2023 Feb 7. WIREs Mech Dis. 2023. PMID: 36750231 Free PMC article. Review.
-
Cannabidiol loaded extracellular vesicles sensitize triple-negative breast cancer to doxorubicin in both in-vitro and in vivo models.Int J Pharm. 2021 Sep 25;607:120943. doi: 10.1016/j.ijpharm.2021.120943. Epub 2021 Jul 27. Int J Pharm. 2021. PMID: 34324983 Free PMC article.
-
Use of Cannabis and Cannabinoids for Treatment of Cancer.Cancers (Basel). 2022 Oct 20;14(20):5142. doi: 10.3390/cancers14205142. Cancers (Basel). 2022. PMID: 36291926 Free PMC article. Review.
-
In Vitro Effect of Δ9-Tetrahydrocannabinol and Cannabidiol on Cancer-Associated Fibroblasts Isolated from Lung Cancer.Int J Mol Sci. 2022 Jun 17;23(12):6766. doi: 10.3390/ijms23126766. Int J Mol Sci. 2022. PMID: 35743206 Free PMC article.
References
-
- Franco-Barraza J., Valdivia-Silva J.E., Zamudio-Meza H., Castillo A., García-Zepeda E.A., Benítez-Bribiesca L., Meza I. Actin Cytoskeleton Participation in the Onset of IL-1β Induction of an Invasive Mesenchymal-like Phenotype in Epithelial MCF-7 Cells. Arch. Med. Res. 2010;41:170–181. doi: 10.1016/j.arcmed.2010.04.010. - DOI - PubMed
-
- Perez-Yepez E.A., Ayala-Sumuano J.-T., Reveles-Espinoza A.M., Meza I. Selection of a MCF-7 Breast Cancer Cell Subpopulation with High Sensitivity to IL-1 β: Characterization of and Correlation between Morphological and Molecular Changes Leading to Increased Invasiveness. Int. J. Breast Cancer. 2012;2012:1–12. doi: 10.1155/2012/609148. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

