Evaluation of the Potential Role of Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) in Niemann-Pick Disease, Type C1
- PMID: 32244519
- PMCID: PMC7178166
- DOI: 10.3390/ijms21072430
Evaluation of the Potential Role of Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) in Niemann-Pick Disease, Type C1
Abstract
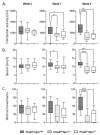
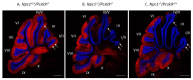
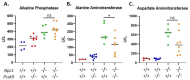
Niemann-Pick disease, type C1, is a cholesterol storage disease where unesterified cholesterol accumulates intracellularly. In the cerebellum this causes neurodegeneration of the Purkinje neurons that die in an anterior-to-posterior and time-dependent manner. This results in cerebellar ataxia as one of the major outcomes of the disease. Proprotein convertase subtilisin/kexin type 9 (PCSK9) plays a significant role in the regulation of serum cholesterol levels by modulating LDL receptor levels on peripheral tissues. In the central nervous system, PCSK9 may have a similar effect on the closely related VLDL and ApoE2 receptors to regulate brain cholesterol. In addition, regulation of VLDLR and ApoER2 by PCSK9 may contribute to neuronal apoptotic pathways through Reelin, the primary ligand of VLDLR and ApoER2. Defects in reelin signaling results in cerebellar dysfunction leading to ataxia as seen in the Reeler mouse. Our recent findings that Pcsk9 is expressed ~8-fold higher in the anterior lobules of the cerebellum compared to the posterior lobule X, which is resistant to neurodegeneration, prompted us to ask whether PCSK9 could play a role in NPC1 disease progression. We addressed this question genetically, by characterizing NPC1 disease in the presence or absence of PCSK9. Analysis of double mutant Pcsk9-/-/Npc1-/- mice by disease severity scoring, motor assessments, lifespan, and cerebellar Purkinje cell staining, showed no obvious difference in NPC1 disease progression with that of Npc1-/- mice. This suggests that PCSK9 does not play an apparent role in NPC1 disease progression.
Keywords: ApoER2; NPC1 KO mouse; Niemann–Pick C; PCSK9; VLDLR; lysosomal storage; neurodegeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Identification of Novel Pathways Associated with Patterned Cerebellar Purkinje Neuron Degeneration in Niemann-Pick Disease, Type C1.Int J Mol Sci. 2019 Dec 31;21(1):292. doi: 10.3390/ijms21010292. Int J Mol Sci. 2019. PMID: 31906248 Free PMC article.
-
Single Cell Transcriptome Analysis of Niemann-Pick Disease, Type C1 Cerebella.Int J Mol Sci. 2020 Jul 28;21(15):5368. doi: 10.3390/ijms21155368. Int J Mol Sci. 2020. PMID: 32731618 Free PMC article.
-
Alteration of GABAergic Input Precedes Neurodegeneration of Cerebellar Purkinje Cells of NPC1-Deficient Mice.Int J Mol Sci. 2019 Dec 13;20(24):6288. doi: 10.3390/ijms20246288. Int J Mol Sci. 2019. PMID: 31847086 Free PMC article.
-
New developments in proprotein convertase subtilisin-kexin 9's biology and clinical implications.Curr Opin Lipidol. 2016 Jun;27(3):274-81. doi: 10.1097/MOL.0000000000000295. Curr Opin Lipidol. 2016. PMID: 27031271 Review.
-
PCSK9 and cancer: Rethinking the link.Biomed Pharmacother. 2021 Aug;140:111758. doi: 10.1016/j.biopha.2021.111758. Epub 2021 May 28. Biomed Pharmacother. 2021. PMID: 34058443 Review.
Cited by
-
PCSK9 Inhibition: From Current Advances to Evolving Future.Cells. 2022 Sep 23;11(19):2972. doi: 10.3390/cells11192972. Cells. 2022. PMID: 36230934 Free PMC article. Review.
-
Complex N-Linked Glycosylation: A Potential Modifier of Niemann-Pick Disease, Type C1 Pathology.Int J Mol Sci. 2022 May 3;23(9):5082. doi: 10.3390/ijms23095082. Int J Mol Sci. 2022. PMID: 35563467 Free PMC article.
-
Organ Weights in NPC1 Mutant Mice Partly Normalized by Various Pharmacological Treatment Approaches.Int J Mol Sci. 2022 Dec 29;24(1):573. doi: 10.3390/ijms24010573. Int J Mol Sci. 2022. PMID: 36614015 Free PMC article.
-
Phenotype assessment for neurodegenerative murine models with ataxia and application to Niemann-Pick disease, type C1.Biol Open. 2022 Apr 15;11(4):bio059052. doi: 10.1242/bio.059052. Epub 2022 May 3. Biol Open. 2022. PMID: 35452076 Free PMC article.
-
Understanding and Treating Niemann-Pick Type C Disease: Models Matter.Int J Mol Sci. 2020 Nov 26;21(23):8979. doi: 10.3390/ijms21238979. Int J Mol Sci. 2020. PMID: 33256121 Free PMC article. Review.
References
-
- Wassif C.A., Cross J.L., Iben J., Sanchez-Pulido L., Cougnoux A., Platt F.M., Ory D.S., Ponting C.P., Bailey-Wilson J.E., Biesecker L.G., et al. High incidence of unrecognized visceral/neurological late-onset Niemann-Pick disease, type C1, predicted by analysis of massively parallel sequencing data sets. Genet. Med. 2016;18:41–48. doi: 10.1038/gim.2015.25. - DOI - PMC - PubMed
-
- Bianconi S.E., Hammond D.I., Farhat N.Y., Dang Do A., Jenkins K., Cougnoux A., Martin K., Porter F.D. Evaluation of age of death in Niemann-Pick disease, type C: Utility of disease support group websites to understand natural history. Mol. Genet. Metab. 2019;126:466–469. doi: 10.1016/j.ymgme.2019.02.004. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

