Exploring the Etiological Links behind Neurodegenerative Diseases: Inflammatory Cytokines and Bioactive Kynurenines
- PMID: 32244523
- PMCID: PMC7177899
- DOI: 10.3390/ijms21072431
Exploring the Etiological Links behind Neurodegenerative Diseases: Inflammatory Cytokines and Bioactive Kynurenines
Abstract
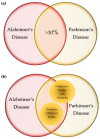
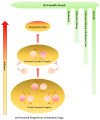
Alzheimer's disease (AD) and Parkinson's disease (PD) are the most common neurodegenerative diseases (NDs), presenting a broad range of symptoms from motor dysfunctions to psychobehavioral manifestations. A common clinical course is the proteinopathy-induced neural dysfunction leading to anatomically corresponding neuropathies. However, current diagnostic criteria based on pathology and symptomatology are of little value for the sake of disease prevention and drug development. Overviewing the pathomechanism of NDs, this review incorporates systematic reviews on inflammatory cytokines and tryptophan metabolites kynurenines (KYNs) of human samples, to present an inferential method to explore potential links behind NDs. The results revealed increases of pro-inflammatory cytokines and neurotoxic KYNs in NDs, increases of anti-inflammatory cytokines in AD, PD, Huntington's disease (HD), Creutzfeldt-Jakob disease, and human immunodeficiency virus (HIV)-associated neurocognitive disorders, and decreases of neuromodulatory KYNs in AD, PD, and HD. The results reinforced a strong link between inflammation and neurotoxic KYNs, confirmed activation of adaptive immune response, and suggested a possible role in the decrease of neuromodulatory KYNs, all of which may contribute to the development of chronic low grade inflammation. Commonalities of multifactorial NDs were discussed to present a current limit of diagnostic criteria, a need for preclinical biomarkers, and an approach to search the initiation factors of NDs.
Keywords: Alzheimer’s disease; Parkinson’s disease; amyloid; cytokine; kynurenine; multifactorial causality; neurodegenerative diseases; neuroinflammation; prion; tau; tryptophan.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

