Molecular Characterization of Tc964, A Novel Antigenic Protein from Trypanosoma cruzi
- PMID: 32244527
- PMCID: PMC7177413
- DOI: 10.3390/ijms21072432
Molecular Characterization of Tc964, A Novel Antigenic Protein from Trypanosoma cruzi
Abstract
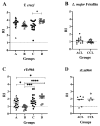
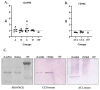
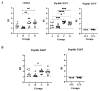
The Tc964 protein was initially identified by its presence in the interactome associated with the LYT1 mRNAs, which code for a virulence factor of Trypanosoma cruzi. Tc964 is annotated in the T. cruzi genome as a hypothetical protein. According to phylogenetic analysis, the protein is conserved in the different genera of the Trypanosomatidae family; however, recognizable orthologues were not identified in other groups of organisms. Therefore, as a first step, an in-depth molecular characterization of the Tc946 protein was carried out. Based on structural predictions and molecular dynamics studies, the Tc964 protein would belong to a particular class of GTPases. Subcellular fractionation analysis indicated that Tc964 is a nucleocytoplasmic protein. Additionally, the protein was expressed as a recombinant protein in order to analyze its antigenicity with sera from Chagas disease (CD) patients. Tc964 was found to be antigenic, and B-cell epitopes were mapped by the use of synthetic peptides. In parallel, the Leishmania major homologue (Lm964) was also expressed as recombinant protein and used for a preliminary evaluation of antigen cross-reactivity in CD patients. Interestingly, Tc964 was recognized by sera from Chronic CD (CCD) patients at different stages of disease severity, but no reactivity against this protein was observed when sera from Colombian patients with cutaneous leishmaniasis were analyzed. Therefore, Tc964 would be adequate for CD diagnosis in areas where both infections (CD and leishmaniasis) coexist, even though additional assays using larger collections of sera are needed in order to confirm its usefulness for differential serodiagnosis.
Keywords: Leishmania major; Trypanosoma cruzi; chagas disease; leishmaniasis; molecular modelling; serodiagnosis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
Cross-reactivity studies and differential serodiagnosis of human infections caused by Trypanosoma cruzi and Leishmania spp; use of immunoblotting and ELISA with a purified antigen (Ag163B6).Clin Exp Immunol. 1994 Sep;97(3):417-23. doi: 10.1111/j.1365-2249.1994.tb06104.x. Clin Exp Immunol. 1994. PMID: 8082296 Free PMC article.
-
Cross-Reactivity Using Chimeric Trypanosoma cruzi Antigens: Diagnostic Performance in Settings Where Chagas Disease and American Cutaneous or Visceral Leishmaniasis Are Coendemic.J Clin Microbiol. 2019 Jul 26;57(8):e00762-19. doi: 10.1128/JCM.00762-19. Print 2019 Aug. J Clin Microbiol. 2019. PMID: 31189586 Free PMC article.
-
[Study of cases of leishmaniasis in the Province of Salta: evidences of mixed infection with Trypanosoma cruzi and Leishmania spp].Medicina (B Aires). 1996;56(3):259-68. Medicina (B Aires). 1996. PMID: 9035482 Spanish.
-
A new Leishmania-specific hypothetical protein and its non-described specific B cell conformational epitope applied in the serodiagnosis of canine visceral leishmaniasis.Parasitol Res. 2016 Apr;115(4):1649-58. doi: 10.1007/s00436-016-4904-x. Epub 2016 Jan 19. Parasitol Res. 2016. PMID: 26782811 Review.
-
Molecular mimicry between Trypanosoma cruzi and host nervous tissues.Acta Cient Venez. 1992;43(6):330-40. Acta Cient Venez. 1992. PMID: 1343745 Review.
Cited by
-
Recombinant proteins as promising antigens applied to the immunodiagnosis of Chagas disease: a scoping review.Front Microbiol. 2024 Jul 30;15:1420226. doi: 10.3389/fmicb.2024.1420226. eCollection 2024. Front Microbiol. 2024. PMID: 39139374 Free PMC article.
-
The use of peptides for immunodiagnosis of human Chagas disease.Amino Acids. 2024 May 2;56(1):35. doi: 10.1007/s00726-024-03394-6. Amino Acids. 2024. PMID: 38698213 Free PMC article. Review.
-
Characterization of Novel Trypanosoma cruzi-Specific Antigen with Potential Use in the Diagnosis of Chagas Disease.Int J Mol Sci. 2024 Jan 18;25(2):1202. doi: 10.3390/ijms25021202. Int J Mol Sci. 2024. PMID: 38256275 Free PMC article.
References
-
- Chagas C. Nova tripanozomiaze humana. Mem. Inst. Oswaldo Cruz. 1909;1:159–218. doi: 10.1590/S0074-02761909000200008. - DOI
-
- Minchin E. A new trypanosome parasitic in human beings. Nature. 1910;84:142–144. doi: 10.1038/084142a0. - DOI
-
- Álvarez-Hernández D.A., Franyuti-Kelly G.A., Díaz López-Silva R., González Chávez A.M., González-Hermosillo D., Vásquez-López R. Chagas disease: Current perspectives on a forgotten disease. Rev. Med. Hosp. Gen. (Mexico) 2016;81:154–164. doi: 10.1016/j.hgmx.2016.09.010. - DOI
-
- World Health Organization (WHO) Chagas Disease (American Trypanosomiasis) [(accessed on 17 April 2019)]; Available online: https://www.who.int/

