Osteoblastic Differentiation on Graphene Oxide-Functionalized Titanium Surfaces: An In Vitro Study
- PMID: 32244572
- PMCID: PMC7221922
- DOI: 10.3390/nano10040654
Osteoblastic Differentiation on Graphene Oxide-Functionalized Titanium Surfaces: An In Vitro Study
Abstract
Background: Titanium implant surfaces are continuously modified to improve biocompatibility and to promote osteointegration. Graphene oxide (GO) has been successfully used to ameliorate biomaterial performances, in terms of implant integration with host tissue. The aim of this study is to evaluate the Dental Pulp Stem Cells (DPSCs) viability, cytotoxic response, and osteogenic differentiation capability in the presence of GO-coated titanium surfaces.
Methods: Two titanium discs types, machined (control, Crtl) and sandblasted and acid-etched (test, Test) discs, were covalently functionalized with GO. The ability of the GO-functionalized substrates to allow the proliferation and differentiation of DPSCs, as well as their cytotoxic potential, were assessed.
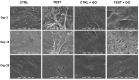
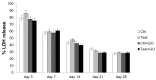
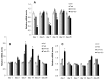
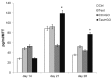
Results: The functionalization procedures provide a homogeneous coating with GO of the titanium surface in both control and test substrates, with unchanged surface roughness with respect to the untreated surfaces. All samples show the deposition of extracellular matrix, more pronounced in the test and GO-functionalized test discs. GO-functionalized test samples evidenced a significant viability, with no cytotoxic response and a remarkable early stage proliferation of DPSCs cells, followed by their successful differentiation into osteoblasts.
Conclusions: The described protocol of GO-functionalization provides a novel not cytotoxic biomaterial that is able to stimulate cell viability and that better and more quickly induces osteogenic differentiation with respect to simple titanium discs. Our findings pave the way to exploit this GO-functionalization protocol for the production of novel dental implant materials that display improved integration with the host tissue.
Keywords: dental pulp stem cells; graphene oxide; osteoblastic differentiation; surface functionalization; titanium disc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





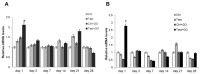
References
-
- Froes F.H. Titanium for medical and dental applications—An introduction. In: Froes F.H., Qian M., editors. Woodhead Publishing Series in Biomaterials, Titanium in Medical and Dental Applications. Woodhead Publishing; Cambridge, UK: 2018. pp. 3–21. - DOI
-
- Iaculli F., Di Filippo E.S., Piattelli A., Mancinelli R., Fulle S. Dental Pulp stem cells grown on dental implant titanium surfaces: An in vitro evaluation of differentiation and microRNAs expression. J. Biomed. Mater. Res. B Appl. Biomater. 2017;105:953–965. doi: 10.1002/jbm.b.33628. - DOI - PubMed
-
- Wennerberg A., Albrektsson T. On implant surfaces: A review of current knowledge and opinions. Int. J. Oral Maxillofac. Implant. 2010;25:63–74. - PubMed
-
- Ferrari A.C., Bonaccorso F., Fal’Ko V., Novoselov K.S., Roche S., Bøggild P., Borini S., Koppens F.H.L., Palermo V., Pugno N., et al. Science and Technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale. 2015;7:4598–4810. doi: 10.1039/C4NR01600A. - DOI - PubMed
Grants and funding
- FAR 2018 Zara/Università degli Studi G. d'Annunzio Chieti - Pescara
- FAR 2018 Cataldi/Università degli Studi G. d'Annunzio Chieti - Pescara
- FAR 2016 Fontana/Università degli Studi G. d'Annunzio Chieti - Pescara
- FAR 2017 Fontana/Università degli Studi G. d'Annunzio Chieti - Pescara
- FAR 2018 Fontana/Università degli Studi G. d'Annunzio Chieti - Pescara
LinkOut - more resources
Full Text Sources

