Properties and Skin Compatibility of Films Based on Poly(Lactic Acid) (PLA) Bionanocomposites Incorporating Chitin Nanofibrils (CN)
- PMID: 32244595
- PMCID: PMC7353621
- DOI: 10.3390/jfb11020021
Properties and Skin Compatibility of Films Based on Poly(Lactic Acid) (PLA) Bionanocomposites Incorporating Chitin Nanofibrils (CN)
Abstract
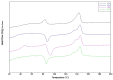
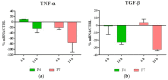
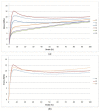
Nanobiocomposites suitable for preparing skin compatible films by flat die extrusion were prepared by using plasticized poly(lactic acid) (PLA), poly(butylene succinate-co-adipate) (PBSA), and Chitin nanofibrils as functional filler. Chitin nanofibrils (CNs) were dispersed in the blends thanks to the preparation of pre-nanocomposites containing poly(ethylene glycol). Thanks to the use of a melt strength enhancer (Plastistrength) and calcium carbonate, the processability and thermal properties of bionanocomposites films containing CNs could be tuned in a wide range. Moreover, the resultant films were flexible and highly resistant. The addition of CNs in the presence of starch proved not advantageous because of an extensive chain scission resulting in low values of melt viscosity. The films containing CNs or CNs and calcium carbonate resulted biocompatible and enabled the production of cells defensins, acting as indirect anti-microbial. Nevertheless, tests made with Staphylococcus aureus and Enterobacter spp. (Gram positive and negative respectively) by the qualitative agar diffusion test did not show any direct anti-microbial activity of the films. The results are explained considering the morphology of the film and the different mechanisms of direct and indirect anti-microbial action generated by the nanobiocomposite based films.
Keywords: anti-microbial; chitin nanofibrils; poly(butylene succinate); poly(lactic acid); skin compatibility; starch.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Sharma R., Jafari S.M., Sharma S. Antimicrobial bio-nanocomposites and their potential applications in food packaging. Food Control. 2020;112:107086. doi: 10.1016/j.foodcont.2020.107086. - DOI
-
- Febo P., Gagliardini A. Baby Diapers Past and Present: A Critical Review. In: Morganti P., editor. Bionanotechnology to Save the Environment: Plant and Fishery’s Biomass as Alternative to Petrol. MDPI; Basel, Switzerland: 2018. pp. 227–238.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

