Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles
- PMID: 32244730
- PMCID: PMC7226770
- DOI: 10.3390/cells9040851
Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles
Abstract
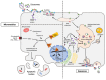
The blood-brain barrier (BBB) is one of the most complex and selective barriers in the human organism. Its role is to protect the brain and preserve the homeostasis of the central nervous system (CNS). The central elements of this physical and physiological barrier are the endothelial cells that form a monolayer of tightly joined cells covering the brain capillaries. However, as endothelial cells regulate nutrient delivery and waste product elimination, they are very sensitive to signals sent by surrounding cells and their environment. Indeed, the neuro-vascular unit (NVU) that corresponds to the assembly of extracellular matrix, pericytes, astrocytes, oligodendrocytes, microglia and neurons have the ability to influence BBB physiology. Extracellular vesicles (EVs) play a central role in terms of communication between cells. The NVU is no exception, as each cell can produce EVs that could help in the communication between cells in short or long distances. Studies have shown that EVs are able to cross the BBB from the brain to the bloodstream as well as from the blood to the CNS. Furthermore, peripheral EVs can interact with the BBB leading to changes in the barrier's properties. This review focuses on current knowledge and potential applications regarding EVs associated with the BBB.
Keywords: blood–brain barrier; brain diseases; exosomes; extracellular vesicles; microvesicles.
Conflict of interest statement
The authors declare no conflicts of interest to disclose.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

