Regulation of the Single Polar Flagellar Biogenesis
- PMID: 32244780
- PMCID: PMC7226244
- DOI: 10.3390/biom10040533
Regulation of the Single Polar Flagellar Biogenesis
Abstract
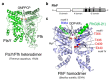
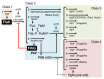
Some bacterial species, such as the marine bacterium Vibrio alginolyticus, have a single polar flagellum that allows it to swim in liquid environments. Two regulators, FlhF and FlhG, function antagonistically to generate only one flagellum at the cell pole. FlhF, a signal recognition particle (SRP)-type guanosine triphosphate (GTP)ase, works as a positive regulator for flagellar biogenesis and determines the location of flagellar assembly at the pole, whereas FlhG, a MinD-type ATPase, works as a negative regulator that inhibits flagellar formation. FlhF intrinsically localizes at the cell pole, and guanosine triphosphate (GTP) binding to FlhF is critical for its polar localization and flagellation. FlhG also localizes at the cell pole via the polar landmark protein HubP to directly inhibit FlhF function at the cell pole, and this localization depends on ATP binding to FlhG. However, the detailed regulatory mechanisms involved, played by FlhF and FlhG as the major factors, remain largely unknown. This article reviews recent studies that highlight the post-translational regulation mechanism that allows the synthesis of only a single flagellum at the cell pole.
Keywords: ATPase; FlaK; FlhF; FlhG; GTPase; HubP; SflA; polar flagellum; protein localization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

