Cloning, Molecular Characterization and Expression Patterns of DMRTC2 Implicated in Germ Cell Development of Male Tibetan Sheep
- PMID: 32244802
- PMCID: PMC7177445
- DOI: 10.3390/ijms21072448
Cloning, Molecular Characterization and Expression Patterns of DMRTC2 Implicated in Germ Cell Development of Male Tibetan Sheep
Abstract
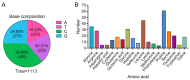
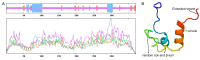
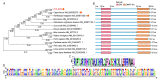
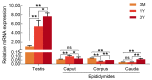
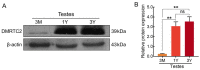
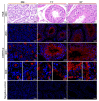
The double sex and mab-3-related transcription factors like family C2 (DMRTC2) gene is indispensable for mammalian testicular function and spermatogenesis. Despite its importance, what expression and roles of DMRTC2 possesses and how it regulates the testicular development and spermatogenesis in sheep, especially in Tibetan sheep, remains largely unknown. In this study, DMRTC2 cDNA from testes of Tibetan sheep was firstly cloned by the RT-PCR method, and its molecular characterization was identified. Subsequently, the expression and localization patterns of DMRTC2 were evaluated by quantitative real-time PCR (qPCR), Western blot, and immunofluorescence. The cloning and sequence analysis showed that the Tibetan sheep DMRTC2 cDNA fragment contained 1113 bp open reading frame (ORF) capable of encoding 370 amino acids, and displayed high identities with some other mammals, which shared an identical DM domain sequence of 47 amino acids ranged from residues 38 to 84. qPCR and Western blot results showed that DMRTC2 was expressed in testes throughout the development stages while not in epididymides (caput, corpus, and cauda), with higher mRNA and protein abundance in Tibetan sheep testes of one- and three-year-old (post-puberty) compared with that of three-month-old (pre-puberty). Immunofluorescence results revealed that immune staining for DMRTC2 protein was observed in spermatids and spermatogonia from post-puberty Tibetan sheep testes, and gonocytes from pre-puberty Tibetan sheep testes. Together, these results demonstrated, for the first time, in sheep, that DMRTC2, as a highly conserved gene in mammals, is essential for sheep spermatogenesis by regulating the proliferation or differentiation of gonocytes and development of spermatids in ram testes at different stages of maturity.
Keywords: DMRTC2; Tibetan sheep; cloning; spermatogenesis; testis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Regulation of GDF9 and CDKN1B expression in Tibetan sheep testes during different stages of maturity.Gene Expr Patterns. 2022 Mar;43:119218. doi: 10.1016/j.gep.2021.119218. Epub 2021 Nov 23. Gene Expr Patterns. 2022. PMID: 34826605
-
Gene expression patterns and protein cellular localization suggest a novel role for DAZL in developing Tibetan sheep testes.Gene. 2020 Mar 20;731:144335. doi: 10.1016/j.gene.2020.144335. Epub 2020 Jan 9. Gene. 2020. PMID: 31927007
-
Characterization of GLOD4 in Leydig Cells of Tibetan Sheep During Different Stages of Maturity.Genes (Basel). 2019 Oct 12;10(10):796. doi: 10.3390/genes10100796. Genes (Basel). 2019. PMID: 31614839 Free PMC article.
-
Identification and functional characterization of developmental-stage-dependent piRNAs in Tibetan sheep testes.J Anim Sci. 2023 Jan 3;101:skad189. doi: 10.1093/jas/skad189. J Anim Sci. 2023. PMID: 37282774 Free PMC article.
-
Differential tissue expression of sex steroid-synthesizing enzyme CYP11A1 in male Tibetan sheep (Ovis aries).Anim Biotechnol. 2023 Dec;34(7):2900-2909. doi: 10.1080/10495398.2022.2125401. Epub 2022 Sep 28. Anim Biotechnol. 2023. PMID: 36169054
Cited by
-
Identification and Expression Pattern of EZH2 in Pig Developing Fetuses.Biomed Res Int. 2020 Oct 5;2020:5315930. doi: 10.1155/2020/5315930. eCollection 2020. Biomed Res Int. 2020. PMID: 33083470 Free PMC article.
-
Mapping the anatomical and transcriptional landscape of early human fetal ovary development.Sci Rep. 2025 May 6;15(1):15814. doi: 10.1038/s41598-025-96135-y. Sci Rep. 2025. PMID: 40328871 Free PMC article.
-
Integrated Analysis of Long Non-Coding RNA and mRNA Expression Profiles in Testes of Calves and Sexually Mature Wandong Bulls (Bos taurus).Animals (Basel). 2021 Jul 5;11(7):2006. doi: 10.3390/ani11072006. Animals (Basel). 2021. PMID: 34359134 Free PMC article.
-
Molecular Characteristics of JAK2 and Its Effect on the Milk Fat and Casein Synthesis of Ovine Mammary Epithelial Cells.Int J Mol Sci. 2024 Apr 4;25(7):4027. doi: 10.3390/ijms25074027. Int J Mol Sci. 2024. PMID: 38612844 Free PMC article.
-
Integrating SNP data to reveal the adaptive selection features of goat populations in extreme environments.BMC Genomics. 2025 Jun 2;26(1):553. doi: 10.1186/s12864-025-11743-2. BMC Genomics. 2025. PMID: 40457191 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

