A Compensatory U1snRNA Partially Rescues FAH Splicing and Protein Expression in a Splicing-Defective Mouse Model of Tyrosinemia Type I
- PMID: 32244944
- PMCID: PMC7139742
- DOI: 10.3390/ijms21062136
A Compensatory U1snRNA Partially Rescues FAH Splicing and Protein Expression in a Splicing-Defective Mouse Model of Tyrosinemia Type I
Abstract
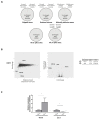
The elucidation of aberrant splicing mechanisms, frequently associated with disease has led to the development of RNA therapeutics based on the U1snRNA, which is involved in 5' splice site (5'ss) recognition. Studies in cellular models have demonstrated that engineered U1snRNAs can rescue different splicing mutation types. However, the assessment of their correction potential in vivo is limited by the scarcity of animal models with the targetable splicing defects. Here, we challenged the U1snRNA in the FAH5961SB mouse model of hepatic fumarylacetoacetate hydrolase (FAH) deficiency (Hereditary Tyrosinemia type I, HT1) due to the FAH c.706G>A splicing mutation. Through minigene expression studies we selected a compensatory U1snRNA (U1F) that was able to rescue this mutation. Intriguingly, adeno-associated virus-mediated delivery of U1F (AAV8-U1F), but not of U1wt, partially rescued FAH splicing in mouse hepatocytes. Consistently, FAH protein was detectable only in the liver of AAV8-U1F treated mice, which displayed a slightly prolonged survival. Moreover, RNA sequencing revealed the negligible impact of the U1F on the splicing profile and overall gene expression, thus pointing toward gene specificity. These data provide early in vivo proof-of-principle of the correction potential of compensatory U1snRNAs in HTI and encourage further optimization on a therapeutic perspective, and translation to other splicing-defective forms of metabolic diseases.
Keywords: FAH; RNA therapeutics; Tyrosinemia type I; U1snRNA; aberrant splicing; fumarylacetoacetate hydrolase deficiency; mouse models.
Conflict of interest statement
D.B., D.S., M.F., S.L., N.Z., C.C., N.P., P.B. and S.F.J.v.d.G. have no competing interests to declare. M.P. is inventor of a patent (PCT/IB2011/054573) on modified U1snRNAs. M.P.had role in the design of the study; in the interpretation of data; in the writing of the manuscript.
Figures


References
-
- Baralle M., Baralle D., De Conti L., Mattocks C., Whittaker J., Knezevich A., Ffrench-Constant C., Baralle F.E. Identification of a mutation that perturbs NF1 gene splicing using genomic DNA samples and a minigene assay. J. Med. Genet. 2003;40:220–222. doi: 10.1136/jmg.40.3.220. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

