Modulation of NLRP3 Inflammasome through Formyl Peptide Receptor 1 (Fpr-1) Pathway as a New Therapeutic Target in Bronchiolitis Obliterans Syndrome
- PMID: 32244997
- PMCID: PMC7139667
- DOI: 10.3390/ijms21062144
Modulation of NLRP3 Inflammasome through Formyl Peptide Receptor 1 (Fpr-1) Pathway as a New Therapeutic Target in Bronchiolitis Obliterans Syndrome
Abstract
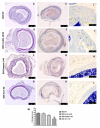
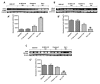
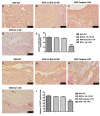
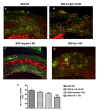
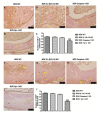
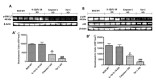
Chronic rejection is the major leading cause of morbidity and mortality after lung transplantation. Bronchiolitis obliterans syndrome (BOS), a fibroproliferative disorder of the small airways, is the main manifestation of chronic lung allograft rejection. We investigated, using transgenic mice, the mechanisms through which the deficiency of IL-1β/IL-18, Casp-1, or Fpr-1 genes could be protective in an experimental model of BOS, induced in mice by allogeneic heterotopic tracheal transplantation. Fpr-1 KO mice showed a marked reduction in histological markers of BOS and of mast cell numbers compared to other groups. Molecular analyses indicated that the absence of the Fpr-1 gene was able to decrease NF-κB nuclear translocation and modulate NLRP3 inflammasome signaling and the mitogen-activated protein kinase (MAPK) pathway in a more significant way compared to other groups. Additionally, Fpr-1 gene deletion caused a reduction in resistance to the apoptosis, assessed by the TUNEL assay. Immunohistochemical analyses indicated changes in nitrotyrosine, PARP, VEGF, and TGF-β expression associated with the pathology, which were reduced in the absence of the Fpr1 gene more so than by the deletion of IL-1β/IL-18 and Casp-1. We underline the importance of the NLRP3 inflammasome and the pathogenic role of Fpr-1 in experimental models of BOS, which is the result of the modulation of immune cell recruitment together with the modulation of local cellular activation, suggesting this gene as a new target in the control of the pathologic features of BOS.
Keywords: bronchiolitis obliterans syndrome; inflammasome; inflammation.
Conflict of interest statement
The authors declare no conflict of interest
Figures

Similar articles
-
Hydrogen-Rich Saline Attenuated Subarachnoid Hemorrhage-Induced Early Brain Injury in Rats by Suppressing Inflammatory Response: Possible Involvement of NF-κB Pathway and NLRP3 Inflammasome.Mol Neurobiol. 2016 Jul;53(5):3462-3476. doi: 10.1007/s12035-015-9242-y. Epub 2015 Jun 20. Mol Neurobiol. 2016. PMID: 26091790
-
MicroRNA-495 Ameliorates Cardiac Microvascular Endothelial Cell Injury and Inflammatory Reaction by Suppressing the NLRP3 Inflammasome Signaling Pathway.Cell Physiol Biochem. 2018;49(2):798-815. doi: 10.1159/000493042. Epub 2018 Aug 30. Cell Physiol Biochem. 2018. PMID: 30165354
-
Critical roles of NLRP3 inflammasome in IL-1β secretion induced by Corynebacterium pseudotuberculosis in vitro.Mol Immunol. 2019 Dec;116:11-17. doi: 10.1016/j.molimm.2019.09.016. Epub 2019 Sep 25. Mol Immunol. 2019. PMID: 31563023
-
The purinergic 2X7 receptor participates in renal inflammation and injury induced by high-fat diet: possible role of NLRP3 inflammasome activation.J Pathol. 2013 Nov;231(3):342-53. doi: 10.1002/path.4237. Epub 2013 Sep 3. J Pathol. 2013. PMID: 23843215
-
MicroRNAs as important regulators of the NLRP3 inflammasome.Prog Biophys Mol Biol. 2020 Jan;150:50-61. doi: 10.1016/j.pbiomolbio.2019.05.004. Epub 2019 May 15. Prog Biophys Mol Biol. 2020. PMID: 31100298 Review.
Cited by
-
Molecular and Biochemical Mechanism of Cannabidiol in the Management of the Inflammatory and Oxidative Processes Associated with Endometriosis.Int J Mol Sci. 2022 May 12;23(10):5427. doi: 10.3390/ijms23105427. Int J Mol Sci. 2022. PMID: 35628240 Free PMC article.
-
Protective effects of Colomast®, A New Formulation of Adelmidrol and Sodium Hyaluronate, in A Mouse Model of Acute Restraint Stress.Int J Mol Sci. 2020 Oct 30;21(21):8136. doi: 10.3390/ijms21218136. Int J Mol Sci. 2020. PMID: 33143356 Free PMC article.
-
Fatty Acid Amide Hydrolase (FAAH) Inhibition Plays a Key Role in Counteracting Acute Lung Injury.Int J Mol Sci. 2022 Mar 3;23(5):2781. doi: 10.3390/ijms23052781. Int J Mol Sci. 2022. PMID: 35269926 Free PMC article.
-
S-Acetyl-Glutathione Attenuates Carbon Tetrachloride-Induced Liver Injury by Modulating Oxidative Imbalance and Inflammation.Int J Mol Sci. 2022 Apr 17;23(8):4429. doi: 10.3390/ijms23084429. Int J Mol Sci. 2022. PMID: 35457246 Free PMC article.
-
The Role of the NLRP3 Inflammasome and Programmed Cell Death in Acute Liver Injury.Int J Mol Sci. 2023 Feb 4;24(4):3067. doi: 10.3390/ijms24043067. Int J Mol Sci. 2023. PMID: 36834481 Free PMC article. Review.
References
-
- Trulock E.P., Christie J.D., Edwards L.B., Boucek M.M., Aurora P., Taylor D.O., Dobbels F., Rahmel A.O., Keck B.M., Hertz M.I. Registry of the international society for heart and lung transplantation: Twenty-fourth official adult lung and heart-lung transplantation report-2007. J. Heart Lung Transpl. 2007;26:782–795. doi: 10.1016/j.healun.2007.06.003. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

