Triple-Negative Breast Cancer: A Review of Conventional and Advanced Therapeutic Strategies
- PMID: 32245065
- PMCID: PMC7143295
- DOI: 10.3390/ijerph17062078
Triple-Negative Breast Cancer: A Review of Conventional and Advanced Therapeutic Strategies
Abstract
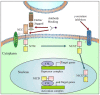
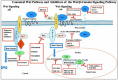
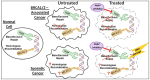
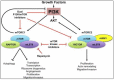
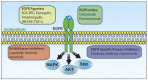
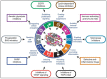
Triple-negative breast cancer (TNBC) cells are deficient in estrogen, progesterone and ERBB2 receptor expression, presenting a particularly challenging therapeutic target due to their highly invasive nature and relatively low response to therapeutics. There is an absence of specific treatment strategies for this tumor subgroup, and hence TNBC is managed with conventional therapeutics, often leading to systemic relapse. In terms of histology and transcription profile these cancers have similarities to BRCA-1-linked breast cancers, and it is hypothesized that BRCA1 pathway is non-functional in this type of breast cancer. In this review article, we discuss the different receptors expressed by TNBC as well as the diversity of different signaling pathways targeted by TNBC therapeutics, for example, Notch, Hedgehog, Wnt/b-Catenin as well as TGF-beta signaling pathways. Additionally, many epidermal growth factor receptor (EGFR), poly (ADP-ribose) polymerase (PARP) and mammalian target of rapamycin (mTOR) inhibitors effectively inhibit the TNBCs, but they face challenges of either resistance to drugs or relapse. The resistance of TNBC to conventional therapeutic agents has helped in the advancement of advanced TNBC therapeutic approaches including hyperthermia, photodynamic therapy, as well as nanomedicine-based targeted therapeutics of drugs, miRNA, siRNA, and aptamers, which will also be discussed. Artificial intelligence is another tool that is presented to enhance the diagnosis of TNBC.
Keywords: artificial intelligence; immunotherapy; nanomedicine; theranostics; triple negative breast cancer.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Breast Cancer Treatment (PDQ(R)): Patient Version. PDQ Adult Treatment Editorial Board; Bethesda, MD, USA: 2002. PDQ Cancer Information Summaries.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

