Detection of Age-Related Changes in Tendon Molecular Composition by Raman Spectroscopy-Potential for Rapid, Non-Invasive Assessment of Susceptibility to Injury
- PMID: 32245089
- PMCID: PMC7139798
- DOI: 10.3390/ijms21062150
Detection of Age-Related Changes in Tendon Molecular Composition by Raman Spectroscopy-Potential for Rapid, Non-Invasive Assessment of Susceptibility to Injury
Abstract
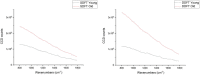
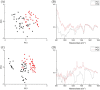
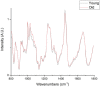
The lack of clinical detection tools at the molecular level hinders our progression in preventing age-related tendon pathologies. Raman spectroscopy can rapidly and non-invasively detect tissue molecular compositions and has great potential for in vivo applications. In biological tissues, a highly fluorescent background masks the Raman spectral features and is usually removed during data processing, but including this background could help age differentiation since fluorescence level in tendons increases with age. Therefore, we conducted a stepwise analysis of fluorescence and Raman combined spectra for better understanding of the chemical differences between young and old tendons. Spectra were collected from random locations of vacuum-dried young and old equine tendon samples (superficial digital flexor tendon (SDFT) and deep digital flexor tendon (DDFT), total n = 15) under identical instrumental settings. The fluorescence-Raman spectra showed an increase in old tendons as expected. Normalising the fluorescence-Raman spectra further indicated a potential change in intra-tendinous fluorophores as tendon ages. After fluorescence removal, the pure Raman spectra demonstrated between-group differences in CH2 bending (1450 cm-1) and various ring-structure and carbohydrate-associated bands (1000-1100 cm-1), possibly relating to a decline in cellular numbers and an accumulation of advanced glycation end products in old tendons. These results demonstrated that Raman spectroscopy can successfully detect age-related tendon molecular differences.
Keywords: Raman spectroscopy; ageing; autofluorescence; collagen; glycation; tendinopathy.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Chemical Markers of Human Tendon Health Identified Using Raman Spectroscopy: Potential for In Vivo Assessment.Int J Mol Sci. 2022 Nov 27;23(23):14854. doi: 10.3390/ijms232314854. Int J Mol Sci. 2022. PMID: 36499181 Free PMC article.
-
Age-related changes to the molecular and cellular components of equine flexor tendons.Equine Vet J. 1999 Sep;31(5):391-6. doi: 10.1111/j.2042-3306.1999.tb03838.x. Equine Vet J. 1999. PMID: 10505954
-
Proteomic analysis reveals age-related changes in tendon matrix composition, with age- and injury-specific matrix fragmentation.J Biol Chem. 2014 Sep 12;289(37):25867-78. doi: 10.1074/jbc.M114.566554. Epub 2014 Jul 30. J Biol Chem. 2014. PMID: 25077967 Free PMC article.
-
Equine flexor tendon imaging part 1: Recent developments in ultrasonography, with focus on the superficial digital flexor tendon.Vet J. 2021 Dec;278:105764. doi: 10.1016/j.tvjl.2021.105764. Epub 2021 Oct 19. Vet J. 2021. PMID: 34678500 Review.
-
Equine flexor tendon imaging part 2: Current status and future directions in advanced diagnostic imaging, with focus on the deep digital flexor tendon.Vet J. 2021 Dec;278:105763. doi: 10.1016/j.tvjl.2021.105763. Epub 2021 Oct 19. Vet J. 2021. PMID: 34678501 Review.
Cited by
-
Raman Spectroscopy in Cellular and Tissue Aging Research.Aging Cell. 2025 Feb;24(2):e14494. doi: 10.1111/acel.14494. Epub 2025 Jan 28. Aging Cell. 2025. PMID: 39876576 Free PMC article. Review.
-
Editorial for Special Issue: Achilles Curse and Remedy: Tendon Diseases from Pathophysiology to Novel Therapeutic Approaches.Int J Mol Sci. 2020 Oct 9;21(20):7454. doi: 10.3390/ijms21207454. Int J Mol Sci. 2020. PMID: 33050349 Free PMC article.
-
Transcriptional Factors Related to Cellular Kinetics, Apoptosis, and Tumorigenicity in Equine Adipose-Derived Mesenchymal Stem Cells (ASCs) Are Influenced by the Age of the Donors.Animals (Basel). 2025 Jun 28;15(13):1910. doi: 10.3390/ani15131910. Animals (Basel). 2025. PMID: 40646808 Free PMC article.
-
Chemical Markers of Human Tendon Health Identified Using Raman Spectroscopy: Potential for In Vivo Assessment.Int J Mol Sci. 2022 Nov 27;23(23):14854. doi: 10.3390/ijms232314854. Int J Mol Sci. 2022. PMID: 36499181 Free PMC article.
-
Individual variation in Achilles tendon morphology and geometry changes susceptibility to injury.Elife. 2021 Feb 16;10:e63204. doi: 10.7554/eLife.63204. Elife. 2021. PMID: 33588992 Free PMC article.
References
-
- Thorpe C.T., Screen H.R.C. Tendon structure and composition. In: Ackermann P.W., Hart D.A., editors. Metabolic Influences on Risk for Tendon Disorders. Springer International Publishing; Cham, Switzerland: 2016. pp. 3–10.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

