Insight Into the Formation Paths of Methyl Bromide From Syringic Acid in Aqueous Bromide Solutions Under Simulated Sunlight Irradiation
- PMID: 32245114
- PMCID: PMC7142905
- DOI: 10.3390/ijerph17062081
Insight Into the Formation Paths of Methyl Bromide From Syringic Acid in Aqueous Bromide Solutions Under Simulated Sunlight Irradiation
Abstract
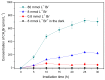
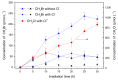
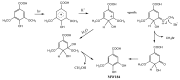
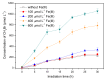
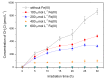
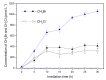
Methyl bromide (CH3Br) is one of the largest natural sources of bromine in the stratosphere, where it leads to ozone depletion. This paper reported the photochemical production of CH3Br from syringic acid (SA) that has been used as an environmentally relevant model compound for terrestrially-derived dissolved organic matter. The formation of CH3Br increased with the increase of bromide ion concentration ranging from 0.8 to 80 mmol L-1. Ferric ions (Fe(III)) enhanced CH3Br production, while chloride inhibited it, with or without Fe(III). Meanwhile, methyl chloride (CH3Cl) was generated in the presence of chloride and was inhibited by Fe(III). The different effects of Fe(III) on the formation of CH3Cl and CH3Br indicate their diverse formation paths. Based on the intermediates identified by liquid chromatography-mass spectrometry and the confirmation of the formation of Fe(III)-SA complexes, it was proposed that there were two formation paths of CH3Br from SA in the bromide-enriched water under simulated sunlight irradiation. One path was via nucleophilic attack of Br- on the excited state protonation of SA; the other was via the combination of methyl radical and bromine radical when Fe(III) was present. This work suggests that the photochemical formation of CH3Br may act as a potential natural source of CH3Br in the bromide-enriched environmental matrix, and helps in better understanding the formation mechanism of CH3Br.
Keywords: methyl bromide; photochemical production; reaction paths; syringic acid.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Methyl bromide production from dissolved organic matter under simulated sunlight irradiation and the important effect of ferric ions.Environ Sci Process Impacts. 2020 Mar 1;22(3):751-758. doi: 10.1039/c9em00473d. Epub 2020 Feb 18. Environ Sci Process Impacts. 2020. PMID: 32067016
-
Methyl bromide: ocean sources, ocean sinks, and climate sensitivity.Global Biogeochem Cycles. 1996 Mar;10(1):175-90. doi: 10.1029/95gb02743. Global Biogeochem Cycles. 1996. PMID: 11539402
-
Methyl Chloride and Methyl Bromide Production and Consumption in Coastal Antarctic Tundra Soils Subject to Sea Animal Activities.Environ Sci Technol. 2020 Oct 20;54(20):13354-13363. doi: 10.1021/acs.est.0c04257. Epub 2020 Oct 8. Environ Sci Technol. 2020. PMID: 32935983
-
Oxidative treatment of bromide-containing waters: formation of bromine and its reactions with inorganic and organic compounds--a critical review.Water Res. 2014 Jan 1;48:15-42. doi: 10.1016/j.watres.2013.08.030. Epub 2013 Sep 4. Water Res. 2014. PMID: 24184020 Review.
-
Formation and control of bromate in sulfate radical-based oxidation processes for the treatment of waters containing bromide: A critical review.Water Res. 2020 Jun 1;176:115725. doi: 10.1016/j.watres.2020.115725. Epub 2020 Mar 16. Water Res. 2020. PMID: 32222545 Review.
References
-
- Schauffler S.M., Atlas E.L., Blake D.R., Flocke F., Lueb R.A., Lee-Taylor J.M., Stroud V., Travnicek W. Distributions of brominated organic compounds in the troposphere and lower stratosphere. J. Geophys. Res. 1999;104:513–535. doi: 10.1029/1999JD900197. - DOI
-
- WMO . Global Ozone Research and Monitoring Project-Report No. 52. WMO; Geneva, Switzerland: 2011. Scientific Assessment of Ozone Depletion: 2010.
-
- Yvon-Lewis S.A., Saltzman E.S., Montzka S.A. Recent trends in atmospheric methyl bromide: Analysis of post-Montreal Protocol variability. Atmos. Chem. Phys. 2009;9:5963–5974. doi: 10.5194/acp-9-5963-2009. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

