Structural Insight into Paramyxovirus and Pneumovirus Entry Inhibition
- PMID: 32245118
- PMCID: PMC7150754
- DOI: 10.3390/v12030342
Structural Insight into Paramyxovirus and Pneumovirus Entry Inhibition
Abstract
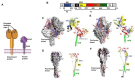
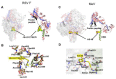
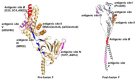
Paramyxoviruses and pneumoviruses infect cells through fusion (F) protein-mediated merger of the viral envelope with target membranes. Members of these families include a range of major human and animal pathogens, such as respiratory syncytial virus (RSV), measles virus (MeV), human parainfluenza viruses (HPIVs), and highly pathogenic Nipah virus (NiV). High-resolution F protein structures in both the metastable pre- and the postfusion conformation have been solved for several members of the families and a number of F-targeting entry inhibitors have progressed to advanced development or clinical testing. However, small-molecule RSV entry inhibitors have overall disappointed in clinical trials and viral resistance developed rapidly in experimental settings and patients, raising the question of whether the available structural information may provide a path to counteract viral escape through proactive inhibitor engineering. This article will summarize current mechanistic insight into F-mediated membrane fusion and examine the contribution of structural information to the development of small-molecule F inhibitors. Implications are outlined for future drug target selection and rational drug engineering strategies.
Keywords: Respiratory syncytial virus; antiviral therapeutic; entry inhibitor; measles virus; nipah virus; parainfluenzavirus; paramyxovirus; pneumovirus; virus entry.
Conflict of interest statement
R.K.P. is a co-inventor on United States patents 8729059 “Paramyxovirus family inhibitors and methods of use thereof” and disclosure filings 20190135770 “Heterocyclic derivatives for the treatment of RSV” and 20190144441 “Bicyclic fused pyrazole derivatives for the treatment of RSV” that cover method of use and composition of matter of the paramyxovirus entry inhibitor class AS-48 and respiratory syncytial virus polymerase inhibitor classes including AWG-233, respectively. This publication could affect his personal financial status. M.A. declares no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Highly Potent Host-Specific Small-Molecule Inhibitor of Paramyxovirus and Pneumovirus Replication with High Resistance Barrier.mBio. 2021 Dec 21;12(6):e0262121. doi: 10.1128/mBio.02621-21. Epub 2021 Nov 2. mBio. 2021. PMID: 34724816 Free PMC article.
-
Third Helical Domain of the Nipah Virus Fusion Glycoprotein Modulates both Early and Late Steps in the Membrane Fusion Cascade.J Virol. 2020 Sep 15;94(19):e00644-20. doi: 10.1128/JVI.00644-20. Print 2020 Sep 15. J Virol. 2020. PMID: 32669342 Free PMC article.
-
Host Cell Restriction Factors of Paramyxoviruses and Pneumoviruses.Viruses. 2020 Dec 2;12(12):1381. doi: 10.3390/v12121381. Viruses. 2020. PMID: 33276587 Free PMC article. Review.
-
Molecular determinants of antiviral potency of paramyxovirus entry inhibitors.J Virol. 2007 Oct;81(19):10567-74. doi: 10.1128/JVI.01181-07. Epub 2007 Jul 25. J Virol. 2007. PMID: 17652384 Free PMC article.
-
Paramyxovirus fusion and entry: multiple paths to a common end.Viruses. 2012 Apr;4(4):613-36. doi: 10.3390/v4040613. Epub 2012 Apr 19. Viruses. 2012. PMID: 22590688 Free PMC article. Review.
Cited by
-
Structure of the Core Postfusion Porcine Endogenous Retrovirus Fusion Protein.mBio. 2022 Feb 22;13(1):e0292021. doi: 10.1128/mbio.02920-21. Epub 2022 Jan 25. mBio. 2022. PMID: 35073741 Free PMC article.
-
Vaccine Development for Human Pneumoviruses.Vaccines (Basel). 2025 May 26;13(6):569. doi: 10.3390/vaccines13060569. Vaccines (Basel). 2025. PMID: 40573900 Free PMC article. Review.
-
Drug repurposing to tackle parainfluenza 3 based on multi-similarities and network proximity analysis.Front Pharmacol. 2024 Oct 1;15:1428925. doi: 10.3389/fphar.2024.1428925. eCollection 2024. Front Pharmacol. 2024. PMID: 39411066 Free PMC article.
-
Helical peptides with disordered regions for measles viruses provide new generalized insights into fusion inhibitors.iScience. 2024 Jan 17;27(2):108961. doi: 10.1016/j.isci.2024.108961. eCollection 2024 Feb 16. iScience. 2024. PMID: 38333694 Free PMC article.
-
The Nucleocapsid of Paramyxoviruses: Structure and Function of an Encapsidated Template.Viruses. 2021 Dec 9;13(12):2465. doi: 10.3390/v13122465. Viruses. 2021. PMID: 34960734 Free PMC article. Review.
References
-
- Lamb R.A., Parks G.D. Paramyxoviridae. In: Knipe D.M., Howley P.M., editors. Fields Virology. 6th ed. Volume 1. Wolters Kluwer/Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2013. pp. 957–995.
-
- Abedi G.R., Prill M.M., Langley G.E., Wikswo M.E., Weinberg G.A., Curns A.T., Schneider E. Estimates of Parainfluenza Virus-Associated Hospitalizations and Cost Among Children Aged Less Than 5 Years in the United States, 1998–2010. J. Pediatric Infect. Dis. Soc. 2016;5:7–13. doi: 10.1093/jpids/piu047. - DOI - PMC - PubMed
-
- Peck A.J., Englund J.A., Kuypers J., Guthrie K.A., Corey L., Morrow R., Hackman R.C., Cent A., Boeckh M. Respiratory virus infection among hematopoietic cell transplant recipients: Evidence for asymptomatic parainfluenza virus infection. Blood. 2007;110:1681–1688. doi: 10.1182/blood-2006-12-060343. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

