Outcomes Associated with Respiratory Failure for Patients with Cardiogenic Shock and Acute Myocardial Infarction: A Substudy of the CULPRIT-SHOCK Trial
- PMID: 32245139
- PMCID: PMC7141492
- DOI: 10.3390/jcm9030860
Outcomes Associated with Respiratory Failure for Patients with Cardiogenic Shock and Acute Myocardial Infarction: A Substudy of the CULPRIT-SHOCK Trial
Abstract
Background: Little is known about clinical outcomes of patients with acute myocardial infraction (AMI) complicated by cardiogenic shock (CS) requiring mechanical ventilation (MV). The aim of this study was to identify the characteristics, risk factors, and outcomes associated with the provision of MV in this specific high-risk population.
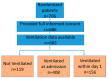
Methods: Patients with CS complicating AMI and multivessel coronary artery disease from the CULPRIT-SHOCK trial were included. We explored 30 days of clinical outcomes in patients not requiring MV, those with MV on admission, and those in whom MV was initiated within the first day after admission.
Results: Among 683 randomized patients included in the analysis, 17.4% received no MV, 59.7% were ventilated at admission and 22.8% received MV within or after the first day after admission. Patients requiring MV had a different risk-profile. Factors independently associated with the provision of MV on admission included higher body weight, resuscitation within 24 h before admission, elevated heart rate and evidence of triple vessel disease.
Conclusions: Requiring MV in patients with CS complicating AMI is common and independently associated with mortality after adjusting for covariates. Patients with delayed MV initiation appear to be at higher risk of adverse outcomes. Further research is necessary to identify the optimal timing of MV in this high-risk population.
Keywords: cardiogenic shock; mechanical ventilation; non-invasive ventilation; respiratory failure.
Conflict of interest statement
Rubini Gimenez reports research grants from Swiss National Foundation (P400PM_180828) for the current work as well as research grants from the Swiss Heart Foundation and speakers’ honoraria from Roche, Ortho Clinical Diagnostics, Abbott and Siemens, outside the submitted work. Miller reports funding through by the Yale National Clinician Scholars Program and by CTSA Grant Number TL1 TR001864 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. Montalescot reports research grants to the Institution or Consulting/Lecture Fees from Abbott, Amgen, Actelion, American College of Cardiology Foundation, AstraZeneca, Axis-Santé, Bayer, Boston-Scientific, Boehringer Ingelheim, Bristol-Myers Squibb, Beth Israel Deaconess Medical, Brigham Women’s Hospital, China heart House, Daiichi-Sankyo, Idorsia, Elsevier, Europa, Fédération Française de Cardiologie, ICAN, Lead-Up, Medtronic, Menarini, MSD, Novo-Nordisk, Partners, Pfizer, Quantum Genomics, Sanofi, Servier, WebMD. Windecker reports research and educational grants to the institution from Abbott, Amgen, Bayer, BMS, Boston Scientific, Biotronik, CSL Behring, Edwards Lifesciences, Medtronic, Polares and Sinom. Oldroyd reports consultancy fees from Abbott Vascular, Boston Scientific and Biosensors and research support from Boston Scientific. de Waha-Thiele reports grants from German Heart Research Foundation, German Cardiac Society, and European Union, Seventh Framework Programme (FP7/2007-2013) Grant agreement n°602202 during the conduct of the study. Zeymer reports grants and personal fees from Astra Zeneca, personal fees from Bayer, grants and personal fees from BMS, grants and personal fees from Daiichi Sankyo, personal fees from Boehringer Ingelheim, personal fees from Medicines Company, personal fees from Sanofi, personal fees from Ferrer, grants and personal fees from Novartis, outside the submitted work. Desch reports grants from European Union during the conduct of the study. Thiele reports grants to the institution from European Union, German Cardiac Society, and German Heart Research Foundation during the conduct of the study.
Figures
References
-
- Helgestad O.K., Josiassen J., Hassager C., Jensen L.O., Holmvang L., Sørensen A., Frydland M., Lassen A.T., Udesen N.L., Schmidt H., et al. Temporal trends in incidence and patient characteristics in cardiogenic shock following acute myocardial infarction from 2010 to 2017: A Danish cohort study. Eur. J. Heart Fail. 2019;21:1370–1378. doi: 10.1002/ejhf.1566. - DOI - PubMed
-
- Alexander J.H., Reynolds H.R., Stebbins A.L., Dzavik V., Harrington R.A., de Werf Van F., Hochman J.S. Effect of tilarginine acetate in patients with acute myocardial infarction and cardiogenic shock: The TRIUMPH randomized controlled trial. JAMA. 2007;297:1657–1666. - PubMed
-
- Hochman J., Sleeper L.A., Webb J.G., A Sanborn T., White H.D., Talley J.D., Buller C.E., Jacobs A.K., Slater J., Col J., et al. Early Revascularization in Acute Myocardial Infarction Complicated by Cardiogenic Shock. N. Engl. J. Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources