Differential Localization of Structural and Non-Structural Proteins during the Bluetongue Virus Replication Cycle
- PMID: 32245145
- PMCID: PMC7150864
- DOI: 10.3390/v12030343
Differential Localization of Structural and Non-Structural Proteins during the Bluetongue Virus Replication Cycle
Abstract
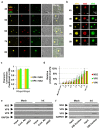
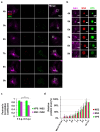
Members of the Reoviridae family assemble virus factories within the cytoplasm of infected cells to replicate and assemble virus particles. Bluetongue virus (BTV) forms virus inclusion bodies (VIBs) that are aggregates of viral RNA, certain viral proteins, and host factors, and have been shown to be sites of the initial assembly of transcriptionally active virus-like particles. This study sought to characterize the formation, composition, and ultrastructure of VIBs, particularly in relation to virus replication. In this study we have utilized various microscopic techniques, including structured illumination microscopy, and virological assays to show for the first time that the outer capsid protein VP5, which is essential for virus maturation, is also associated with VIBs. The addition of VP5 to assembled virus cores exiting VIBs is required to arrest transcriptionally active core particles, facilitating virus maturation. Furthermore, we observed a time-dependent association of the glycosylated non-structural protein 3 (NS3) with VIBs, and report on the importance of the two polybasic motifs within NS3 that facilitate virus trafficking and egress from infected cells at the plasma membrane. Thus, the presence of VP5 and the dynamic nature of NS3 association with VIBs that we report here provide novel insight into these previously less well-characterized processes.
Keywords: bluetongue virus; viral inclusion bodies; virus egress; virus maturation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Bluetongue Virus Nonstructural Protein 3 Orchestrates Virus Maturation and Drives Non-Lytic Egress via Two Polybasic Motifs.Viruses. 2019 Nov 29;11(12):1107. doi: 10.3390/v11121107. Viruses. 2019. PMID: 31795485 Free PMC article.
-
Characterization of virus inclusion bodies in bluetongue virus-infected cells.J Gen Virol. 1993 Mar;74 ( Pt 3):525-30. doi: 10.1099/0022-1317-74-3-525. J Gen Virol. 1993. PMID: 8383185
-
Bluetongue virus RNA binding protein NS2 is a modulator of viral replication and assembly.BMC Mol Biol. 2007 Jan 22;8:4. doi: 10.1186/1471-2199-8-4. BMC Mol Biol. 2007. PMID: 17241458 Free PMC article.
-
Bluetongue virus capsid assembly and maturation.Viruses. 2014 Aug 21;6(8):3250-70. doi: 10.3390/v6083250. Viruses. 2014. PMID: 25196482 Free PMC article. Review.
-
Functional mapping of bluetongue virus proteins and their interactions with host proteins during virus replication.Cell Biochem Biophys. 2008;50(3):143-57. doi: 10.1007/s12013-008-9009-4. Epub 2008 Feb 26. Cell Biochem Biophys. 2008. PMID: 18299997 Review.
Cited by
-
RNA genome packaging and capsid assembly of bluetongue virus visualized in host cells.Cell. 2024 Apr 25;187(9):2236-2249.e17. doi: 10.1016/j.cell.2024.03.007. Epub 2024 Apr 12. Cell. 2024. PMID: 38614100 Free PMC article.
-
Multiple Routes of Bluetongue Virus Egress.Microorganisms. 2020 Jun 27;8(7):965. doi: 10.3390/microorganisms8070965. Microorganisms. 2020. PMID: 32605099 Free PMC article. Review.
-
Role of NS2 specific RNA binding and phosphorylation in liquid-liquid phase separation and virus assembly.Nucleic Acids Res. 2022 Oct 28;50(19):11273-11284. doi: 10.1093/nar/gkac904. Nucleic Acids Res. 2022. PMID: 36259663 Free PMC article.
-
Bluetongue virus assembly and exit pathways.Adv Virus Res. 2020;108:249-273. doi: 10.1016/bs.aivir.2020.08.002. Epub 2020 Sep 16. Adv Virus Res. 2020. PMID: 33837718 Free PMC article. Review.
-
Inhibition of the IFN Response by Bluetongue Virus: The Story So Far.Front Microbiol. 2021 Jun 8;12:692069. doi: 10.3389/fmicb.2021.692069. eCollection 2021. Front Microbiol. 2021. PMID: 34168637 Free PMC article. Review.
References
-
- Ratinier M., Caporale M., Golder M., Franzoni G., Allan K., Nunes S.F., Armezzani A., Bayoumy A., Rixon F., Shaw A., et al. Identification and characterization of a novel non-structural protein of bluetongue virus. PLoS Pathog. 2011;7:e1002477. doi: 10.1371/journal.ppat.1002477. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

