Antibody-Drug Conjugate Using Ionized Cys-Linker-MMAE as the Potent Payload Shows Optimal Therapeutic Safety
- PMID: 32245171
- PMCID: PMC7140114
- DOI: 10.3390/cancers12030744
Antibody-Drug Conjugate Using Ionized Cys-Linker-MMAE as the Potent Payload Shows Optimal Therapeutic Safety
Abstract
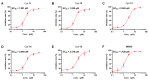
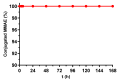
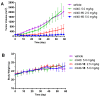
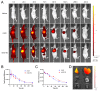
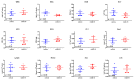
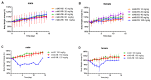
Monomethyl auristatin E (MMAE) is the most popular and widely used cytotoxin in the development of antibody-drug conjugates (ADCs). However, current MMAE-based ADCs are all constructed using cleavable linkers, and this design concept still has insurmountable drawbacks. Their potential instabilities and lipophilic MMAE-induced "bystander effect" inevitably increase the toxicity to normal tissues. Herein, we overturn previous negative views of MMAE-based ADCs with non-cleavable linkers and propose using ionized L-Cysteine (Cys)-linker-MMAE as a novel payload, which can ingeniously enrich and enter tumor cells through receptor-mediated endocytosis of antibodies while its lower permeability helps to avoid further off-target toxicity. We demonstrate that Cys-linker-MMAE maintains high potency similar to free MMAE at the tubulin molecular level and can also be efficiently released in target cells. As a result, the preferred ADC (mil40-15) not only exhibits ideal plasma stability and maintains potent cytotoxicity as MMAE (IC50: 10-11 M), but also shows improved safety with lower bystander toxicity (IC50: 10-9 M), its maximum tolerated dose approaching the level of the naked antibody (160 mg/kg). This study indicated that Cys-linker-MMAE has the potential as a potent payload for ADCs, which is expected to provide novel strategies for the development of MMAE-based ADCs.
Keywords: Cys-linker-MMAE; MMAE; antibody-drug conjugate; linker; tumor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

