Non-Coding RNAs and Their Role in Respiratory Syncytial Virus (RSV) and Human Metapneumovirus (hMPV) Infections
- PMID: 32245206
- PMCID: PMC7150941
- DOI: 10.3390/v12030345
Non-Coding RNAs and Their Role in Respiratory Syncytial Virus (RSV) and Human Metapneumovirus (hMPV) Infections
Abstract
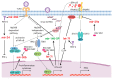
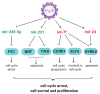
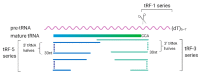
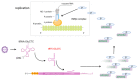
Recent high-throughput sequencing revealed that only 2% of the transcribed human genome codes for proteins, while the majority of transcriptional products are non-coding RNAs (ncRNAs). Herein, we review the current knowledge regarding ncRNAs, both host- and virus-derived, and their role in respiratory syncytial virus (RSV) and human metapneumovirus (hMPV) infections. RSV is known as the most common cause of lower respiratory tract infection (LRTI) in children, while hMPV is also a significant contributor to LRTI in the pediatrics population. Although RSV and hMPV are close members, belonging to the Pneumoviridae family, they induce distinct changes in the ncRNA profile. Several types of host ncRNAs, including long ncRNA (lncRNA), microRNAs (miRNAs), and transfer RNA (tRNA)-derived RNA fragments (tRFs), are involved as playing roles in RSV and/or hMPV infection. Given the importance of ncRNAs in regulating the expression and functions of genes and proteins, comprehensively understanding the roles of ncRNAs in RSV/hMPV infection could shed light upon the disease mechanisms of RSV and hMPV, potentially providing insights into the development of prevention strategies and antiviral therapy. The presence of viral-derived RNAs and the potential of using ncRNAs as diagnostic biomarkers are also discussed in this review.
Keywords: RSV; hMPV; ncRNAs.
Conflict of interest statement
All authors concur there are no conflicts of interest associated with this published work. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Comparing Human Metapneumovirus and Respiratory Syncytial Virus: Viral Co-Detections, Genotypes and Risk Factors for Severe Disease.PLoS One. 2017 Jan 17;12(1):e0170200. doi: 10.1371/journal.pone.0170200. eCollection 2017. PLoS One. 2017. PMID: 28095451 Free PMC article.
-
RSV and HMPV Infections in 3D Tissue Cultures: Mechanisms Involved in Virus-Host and Virus-Virus Interactions.Viruses. 2021 Jan 19;13(1):139. doi: 10.3390/v13010139. Viruses. 2021. PMID: 33478119 Free PMC article.
-
Clinical characteristics and viral load of respiratory syncytial virus and human metapneumovirus in children hospitaled for acute lower respiratory tract infection.J Med Virol. 2017 Apr;89(4):589-597. doi: 10.1002/jmv.24687. Epub 2016 Sep 28. J Med Virol. 2017. PMID: 27632796 Free PMC article.
-
Perspective on the host response to human metapneumovirus infection: what can we learn from respiratory syncytial virus infections?Microbes Infect. 2006 Jan;8(1):285-93. doi: 10.1016/j.micinf.2005.07.001. Epub 2005 Aug 10. Microbes Infect. 2006. PMID: 16182587 Free PMC article. Review.
-
The distinguishing features of human metapneumovirus and respiratory syncytial virus.Rev Med Virol. 2010 Jul;20(4):245-60. doi: 10.1002/rmv.651. Rev Med Virol. 2010. PMID: 20586081 Review.
Cited by
-
The role of human ribonuclease A family in health and diseases: A systematic review.iScience. 2022 Oct 7;25(11):105284. doi: 10.1016/j.isci.2022.105284. eCollection 2022 Nov 18. iScience. 2022. PMID: 36304117 Free PMC article. Review.
-
Immune escaping of the novel genotypes of human respiratory syncytial virus based on gene sequence variation.Front Immunol. 2023 Jan 10;13:1084139. doi: 10.3389/fimmu.2022.1084139. eCollection 2022. Front Immunol. 2023. PMID: 36703972 Free PMC article.
-
The emerging role of microRNAs in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.Int Immunopharmacol. 2021 Jan;90:107204. doi: 10.1016/j.intimp.2020.107204. Epub 2020 Nov 13. Int Immunopharmacol. 2021. PMID: 33221169 Free PMC article. Review.
-
Host tRNA-Derived RNAs Target the 3'Untranslated Region of SARS-CoV-2.Pathogens. 2022 Dec 6;11(12):1479. doi: 10.3390/pathogens11121479. Pathogens. 2022. PMID: 36558813 Free PMC article.
-
Inhibition of Respiratory Syncytial Virus Infection by Small Non-Coding RNA Fragments.Int J Mol Sci. 2022 May 26;23(11):5990. doi: 10.3390/ijms23115990. Int J Mol Sci. 2022. PMID: 35682669 Free PMC article.
References
-
- Peter F., Wright F.T.C. Generic Protocol to Examine the Incidence of Lower Respiratory Infection due to Respiratory Syncytial Virus in Children Less Than Five Years of Age. World Health Organization, Department of Vaccines and Biologicals; Geneva, Switzerland: 2000.
-
- Henderson J., Hilliard T.N., Sherriff A., Stalker D., Al Shammari N., Thomas H.M. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: A longitudinal birth cohort study. Pediatr Allergy Immunol. 2005;16:386–392. doi: 10.1111/j.1399-3038.2005.00298.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

