Degeneration of Lumbar Intervertebral Discs: Characterization of Anulus Fibrosus Tissue and Cells of Different Degeneration Grades
- PMID: 32245213
- PMCID: PMC7139657
- DOI: 10.3390/ijms21062165
Degeneration of Lumbar Intervertebral Discs: Characterization of Anulus Fibrosus Tissue and Cells of Different Degeneration Grades
Abstract
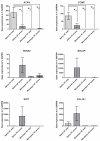
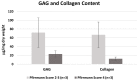
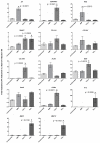
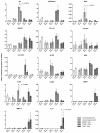
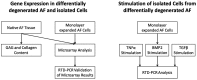
Intervertebral disc (IVD) herniation and degeneration is a major source of back pain. In order to regenerate a herniated and degenerated disc, closure of the anulus fibrosus (AF) is of crucial importance. For molecular characterization of AF, genome-wide Affymetrix HG-U133plus2.0 microarrays of native AF and cultured cells were investigated. To evaluate if cells derived from degenerated AF are able to initiate gene expression of a regenerative pattern of extracellular matrix (ECM) molecules, cultivated cells were stimulated with bone morphogenetic protein 2 (BMP2), transforming growth factor β1 (TGFβ1) or tumor necrosis factor-α (TNFα) for 24 h. Comparative microarray analysis of native AF tissues showed 788 genes with a significantly different gene expression with 213 genes more highly expressed in mild and 575 genes in severe degenerated AF tissue. Mild degenerated native AF tissues showed a higher gene expression of common cartilage ECM genes, whereas severe degenerated AF tissues expressed genes known from degenerative processes, including matrix metalloproteinases (MMP) and bone associated genes. During monolayer cultivation, only 164 differentially expressed genes were found. The cells dedifferentiated and altered their gene expression profile. RTD-PCR analyses of BMP2- and TGFβ1-stimulated cells from mild and severe degenerated AF tissue after 24 h showed an increased expression of cartilage associated genes. TNFα stimulation increased MMP1, 3, and 13 expression. Cells derived from mild and severe degenerated tissues could be stimulated to a comparable extent. These results give hope that regeneration of mildly but also strongly degenerated disc tissue is possible.
Keywords: anulus fibrosus; degeneration; genome-wide microarray; intervertebral disc.
Conflict of interest statement
J.-P.K. and M.E. are employees of TransTissue Technologies GmbH (TTT). TTT develops regenerative medicine products based on resorbable scaffolds. The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Schmidt C.O., Raspe H., Pfingsten M., Hasenbring M., Basler H.D., Eich W., Kohlmann T. Back pain in the German adult population: Prevalence, severity, and sociodemographic correlates in a multiregional survey. Spine (Phila Pa 1976) 2007;32:2005–2011. doi: 10.1097/BRS.0b013e318133fad8. - DOI - PubMed
-
- Lebow R.L., Adogwa O., Parker S.L., Sharma A., Cheng J., McGirt M.J. Asymptomatic same-site recurrent disc herniation after lumbar discectomy: Results of a prospective longitudinal study with 2-year serial imaging. Spine (Phila Pa 1976) 2011;36:2147–2151. doi: 10.1097/BRS.0b013e3182054595. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

