Structural Requirements of Benzofuran Derivatives Dehydro- δ- and Dehydro- ε-Viniferin for Antimicrobial Activity Against the Foodborne Pathogen Listeria monocytogenes
- PMID: 32245220
- PMCID: PMC7139904
- DOI: 10.3390/ijms21062168
Structural Requirements of Benzofuran Derivatives Dehydro- δ- and Dehydro- ε-Viniferin for Antimicrobial Activity Against the Foodborne Pathogen Listeria monocytogenes
Abstract
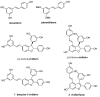
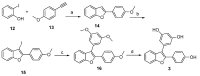
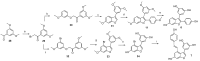
In a recent study, we investigated the antimicrobial activity of a collection of resveratrol-derived monomers and dimers against a series of foodborne pathogens. Out of the tested molecules, dehydro-δ-viniferin and dehydro-ε-viniferin emerged as the most promising derivatives. To define the structural elements essential to the antimicrobial activity against the foodborne pathogen L. monocytogenes Scott A as a model Gram-positive microorganism, the synthesis of a series of simplified benzofuran-containing derivatives was carried out. The systematic removal of the aromatic moieties of the parent molecules allowed a deeper insight into the most relevant structural features affecting the activity. While the overall structure of compound 1 could not be altered without a substantial loss of antimicrobial activity, the structural simplification of compound 2 (minimal inhibitory concentration (MIC) 16 µg/mL, minimal bactericidal concentration (MBC) >512 µg/mL) led to the analogue 7 with increased activity (MIC 8 µg/mL, MBC 64 µg/mL).
Keywords: Listeria monocytogenes; antimicrobials; benzofuran nucleus; viniferin derivatives.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Synthesis and Antimicrobial Activity of δ-Viniferin Analogues and Isosteres.Molecules. 2021 Dec 15;26(24):7594. doi: 10.3390/molecules26247594. Molecules. 2021. PMID: 34946674 Free PMC article.
-
Antimicrobial activity of resveratrol-derived monomers and dimers against foodborne pathogens.Sci Rep. 2019 Dec 20;9(1):19525. doi: 10.1038/s41598-019-55975-1. Sci Rep. 2019. PMID: 31862939 Free PMC article.
-
Wild Vitis Species as Stilbenes Sources: Cane Extracts and Their Antibacterial Activity against Listeria monocytogenes.Molecules. 2024 Jul 26;29(15):3518. doi: 10.3390/molecules29153518. Molecules. 2024. PMID: 39124922 Free PMC article.
-
In the shadow of resveratrol: biological activities of epsilon-viniferin.J Physiol Biochem. 2022 May;78(2):465-484. doi: 10.1007/s13105-022-00880-x. Epub 2022 Mar 21. J Physiol Biochem. 2022. PMID: 35312966 Review.
-
Chemistry, Biosynthesis and Pharmacology of Viniferin: Potential Resveratrol-Derived Molecules for New Drug Discovery, Development and Therapy.Molecules. 2022 Aug 9;27(16):5072. doi: 10.3390/molecules27165072. Molecules. 2022. PMID: 36014304 Free PMC article. Review.
Cited by
-
Stilbenoids: A Natural Arsenal against Bacterial Pathogens.Antibiotics (Basel). 2020 Jun 18;9(6):336. doi: 10.3390/antibiotics9060336. Antibiotics (Basel). 2020. PMID: 32570824 Free PMC article. Review.
-
Natural and nature-inspired stilbenoids as antiviral agents.Eur J Med Chem. 2020 Sep 15;202:112541. doi: 10.1016/j.ejmech.2020.112541. Epub 2020 Jul 4. Eur J Med Chem. 2020. PMID: 32652408 Free PMC article. Review.
-
Cytotoxicity of Benzofuran-Containing Simplified Viniferin Analogues.Pharmaceuticals (Basel). 2024 Aug 1;17(8):1012. doi: 10.3390/ph17081012. Pharmaceuticals (Basel). 2024. PMID: 39204117 Free PMC article.
-
Generation of potent antibacterial compounds through enzymatic and chemical modifications of the trans-δ-viniferin scaffold.Sci Rep. 2023 Sep 25;13(1):15986. doi: 10.1038/s41598-023-43000-5. Sci Rep. 2023. PMID: 37749179 Free PMC article.
-
Progress of Antimicrobial Mechanisms of Stilbenoids.Pharmaceutics. 2024 May 15;16(5):663. doi: 10.3390/pharmaceutics16050663. Pharmaceutics. 2024. PMID: 38794325 Free PMC article. Review.
References
-
- Hiremathad A., Patil M.R., Chethana K.R., Chand K., Amelia Santos M., Keri R.S. Benzofuran: An emerging scaffold for antimicrobial agents. RSC Adv. 2015;5:96809–96828. doi: 10.1039/C5RA20658H. - DOI

