Evaluation of Cyprinid Herpesvirus 2 Latency and Reactivation in Carassius gibel
- PMID: 32245260
- PMCID: PMC7143840
- DOI: 10.3390/microorganisms8030445
Evaluation of Cyprinid Herpesvirus 2 Latency and Reactivation in Carassius gibel
Abstract
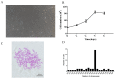
Cyprinid herpesvirus 2 (CyHV-2, species Cyprinid herpesvirus 2) causes severe mortality in ornamental goldfish, crucian carp (Carassius auratus), and gibel carp (Carassius gibelio). It has been shown that the genomic DNA of CyHV-2 could be detected in subclinical fish, which implied that CyHV-2 could establish persistent infection. In this study, the latency of CyHV-2 was investigated in the survival fish after primary infection. CyHV-2 genomic DNA was detected in multiple tissues of acute infection samples; however, detection of CyHV-2 DNA was significantly reduced in fish recovered from the primary infection on day 300 postinfection. No active viral gene transcription, such as DNA polymerase and ORF99, was detected in recovered fish. Following temperature stress, an increase of CyHV-2 DNA copy numbers and gene transcription were observed in tissues examined, which suggests that CyHV-2 was reactivated under stress. In addition, a cell line (GCBLat1) derived from the brain tissue from CyHV-2-exposed fish harbored CyHV-2 genome but did not produce infectious virions under normal culture conditions. However, CyHV-2 replication and viral gene transcription occurred when GCBLat1 cells were treated with trichostatin A (TSA) or phorbol 12-myristate 13-acetate (TPA). It suggests CyHV-2 can remain latent in vitro and can reactivate under stress condition.
Keywords: Cyprinid herpesvirus 2; cell line; gibel carp; latency; reactivation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Hedrick R.P., Waltzek T.B., Mcdowell T.S. Susceptibility of Koi Carp, Common Carp, Goldfish, and Goldfish × Common Carp Hybrids to Cyprinid Herpesvirus2 and Herpesvirus3. J. Aquat. Anim. Health. 2006;18:26–34. doi: 10.1577/H05-028.1. - DOI
-
- Fichi G., Cardeti G., Cocumelli C., Vendramin N., Susini F. Detection of Cyprinid herpesvirus 2 in association with an Aeromonas sobria infection of Carassius carassius (L.), in Italy. J. Fish Dis. 2013;36:823–830. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

