Off-label and unlicensed drug use in Ayder comprehensive specialized hospital neonatal intensive care unit
- PMID: 32245504
- PMCID: PMC7118957
- DOI: 10.1186/s13052-020-0809-5
Off-label and unlicensed drug use in Ayder comprehensive specialized hospital neonatal intensive care unit
Abstract
Background: Off- label drug use refers to the use of medicines outside of their marketing authorization with respect to dose, dosage form, route of administration, indication or age. Off-label/unlicensed drug use significantly associated with adverse drug reactions and medication errors in neonates and critically ill neonates are more vulnerable to these problems.
Objective: To assess the prevalence and associated factors with off-label and unlicensed drug use in neonatal intensive care unit of Ayder Comprehensive Specialized Hospital.
Methods: A cross-sectional study was conducted from March 01,2019 to April 30, 2019 in neonatal intensive care unit of Ayder Comprehensive Specialized Hospital. Neonates admitted for 24 h and took at least one medicine were included in the study. Data was collected from prescription and medical charts. The off-label and license status of the medicine was verified based on European medicine Agency electronic medicine compendium. Data was analyzed by SPSS version 21.0. Binary and multivariate logistic regression was done to assess the predictors of off-label/unlicensed medicine use at p-value ≤0.05 significance level.
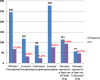
Result: A total of 364 medicines prescribed for 122 neonates were analyzed. The prevalence of off-label and unlicensed drug use was 246 (67.58%), and 86 (23.63%) respectively. Of the total 122 neonates, 114(93.44%), and 57(46.72%) of them were exposed to at least one off-label and unlicensed drug respectively. Antibiotics were the most commonly prescribed off-label and unlicensed drugs. No statistically significant association was found between demographic as well as health related variables with off-label/unlicensed medicine use at p-value of ≤0.05 significance level.
Conclusion: Off-label and unlicensed medicine use was high among neonates admitted to intensive care unit of the hospital. Selecting the safest medicines for such vulnerable patients is crucial to promote rational prescribing and better therapeutic benefit.
Keywords: Drug-use; Intensive care unit; Neonate; Off-label use; Unlicensed use.
Conflict of interest statement
All authors declared that there is no competing interest.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous