Engineered Reporter Phages for Rapid Bioluminescence-Based Detection and Differentiation of Viable Listeria Cells
- PMID: 32245761
- PMCID: PMC7237785
- DOI: 10.1128/AEM.00442-20
Engineered Reporter Phages for Rapid Bioluminescence-Based Detection and Differentiation of Viable Listeria Cells
Abstract
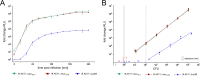
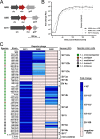
The pathogen Listeria monocytogenes causes listeriosis, a severe foodborne disease associated with high mortality. Rapid and sensitive methods are required for specific detection of this pathogen during food production. Bioluminescence-based reporter bacteriophages are genetically engineered viruses that infect their host cells with high specificity and transduce a heterologous luciferase gene whose activity can be detected with high sensitivity to indicate the presence of viable target cells. Here, we use synthetic biology for de novo genome assembly and activation as well as CRISPR-Cas-assisted phage engineering to construct a set of reporter phages for the detection and differentiation of viable Listeria cells. Based on a single phage backbone, we compare the performance of four reporter phages that encode different crustacean, cnidarian, and bacterial luciferases. From this panel of reporter proteins, nanoluciferase (NLuc) was identified as a superior enzyme and was subsequently introduced into the genomes of a broad host range phage (A511) and two serovar 1/2- and serovar 4b/6a-specific Listeria phages (A006 and A500, respectively). The broad-range NLuc-based phage A511::nlucCPS detects one CFU of L. monocytogenes in 25 g of artificially contaminated milk, cold cuts, and lettuce within less than 24 h. In addition, this reporter phage successfully detected Listeria spp. in potentially contaminated natural food samples without producing false-positive or false-negative results. Finally, A006::nluc and A500::nluc enable serovar-specific Listeria diagnostics. In conclusion, these NLuc-based reporter phages enable rapid, ultrasensitive detection and differentiation of viable Listeria cells using a simple protocol that is 72 h faster than culture-dependent approaches.IMPORTANCE Culture-dependent methods are the gold standard for sensitive and specific detection of pathogenic bacteria within the food production chain. In contrast to molecular approaches, these methods detect viable cells, which is a key advantage for foods generated from heat-inactivated source material. However, culture-based diagnostics are typically much slower than molecular or proteomic strategies. Reporter phage assays combine the best of both worlds and allow for near online assessment of microbial safety because phage replication is extremely fast, highly target specific, and restricted to metabolically active host cells. In addition, reporter phage assays are inexpensive and do not require highly trained personnel, facilitating their on-site implementation. The reporter phages presented in this study not only allow for rapid detection but also enable an early estimation of the potential virulence of Listeria isolates from food production and processing sites.
Keywords: CRISPR-Cas; Listeria monocytogenes; bacteriophage; bioluminescence; food safety; phage engineering; reporter phage; synthetic biology.
Copyright © 2020 Meile et al.
Figures



Similar articles
-
Evaluation of luciferase reporter bacteriophage A511::luxAB for detection of Listeria monocytogenes in contaminated foods.Appl Environ Microbiol. 1997 Aug;63(8):2961-5. doi: 10.1128/aem.63.8.2961-2965.1997. Appl Environ Microbiol. 1997. PMID: 9251182 Free PMC article.
-
Ultrasensitive and Fast Diagnostics of Viable Listeria Cells by CBD Magnetic Separation Combined with A511::luxAB Detection.Viruses. 2018 Nov 13;10(11):626. doi: 10.3390/v10110626. Viruses. 2018. PMID: 30428537 Free PMC article.
-
Construction of luciferase reporter bacteriophage A511::luxAB for rapid and sensitive detection of viable Listeria cells.Appl Environ Microbiol. 1996 Apr;62(4):1133-40. doi: 10.1128/aem.62.4.1133-1140.1996. Appl Environ Microbiol. 1996. PMID: 8919773 Free PMC article.
-
Reporter Phage-Based Detection of Bacterial Pathogens: Design Guidelines and Recent Developments.Viruses. 2020 Aug 26;12(9):944. doi: 10.3390/v12090944. Viruses. 2020. PMID: 32858938 Free PMC article. Review.
-
Detection of bacteria with bioluminescent reporter bacteriophage.Adv Biochem Eng Biotechnol. 2014;144:155-71. doi: 10.1007/978-3-662-43385-0_5. Adv Biochem Eng Biotechnol. 2014. PMID: 25084997 Review.
Cited by
-
Genetic Engineering and Rebooting of Bacteriophages in L-Form Bacteria.Methods Mol Biol. 2024;2734:247-259. doi: 10.1007/978-1-0716-3523-0_16. Methods Mol Biol. 2024. PMID: 38066374
-
The Age of Phage: Friend or Foe in the New Dawn of Therapeutic and Biocontrol Applications?Pharmaceuticals (Basel). 2021 Feb 28;14(3):199. doi: 10.3390/ph14030199. Pharmaceuticals (Basel). 2021. PMID: 33670836 Free PMC article. Review.
-
Engineered bacteriophages: A panacea against pathogenic and drug resistant bacteria.Heliyon. 2024 Jul 9;10(14):e34333. doi: 10.1016/j.heliyon.2024.e34333. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39100447 Free PMC article. Review.
-
How Broad Is Enough: The Host Range of Bacteriophages and Its Impact on the Agri-Food Sector.Phage (New Rochelle). 2021 Jun 1;2(2):83-91. doi: 10.1089/phage.2020.0036. Epub 2021 Jun 16. Phage (New Rochelle). 2021. PMID: 36148040 Free PMC article. Review.
-
L-form conversion in Gram-positive bacteria enables escape from phage infection.Nat Microbiol. 2023 Mar;8(3):387-399. doi: 10.1038/s41564-022-01317-3. Epub 2023 Jan 30. Nat Microbiol. 2023. PMID: 36717719 Free PMC article.
References
-
- Buchanan RL, Gorris LGM, Hayman MM, Jackson TC, Whiting RC. 2017. A review of Listeria monocytogenes: an update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 75:1–13. doi:10.1016/j.foodcont.2016.12.016. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

