Dual-Localized Enzymatic Components Constitute the Fatty Acid Synthase Systems in Mitochondria and Plastids
- PMID: 32245791
- PMCID: PMC7271793
- DOI: 10.1104/pp.19.01564
Dual-Localized Enzymatic Components Constitute the Fatty Acid Synthase Systems in Mitochondria and Plastids
Abstract
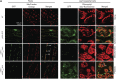
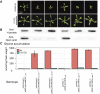
Plant fatty acid biosynthesis occurs in both plastids and mitochondria. Here, we report the identification and characterization of Arabidopsis (Arabidopsis thaliana) genes encoding three enzymes shared between the mitochondria- and plastid-localized type II fatty acid synthase systems (mtFAS and ptFAS, respectively). Two of these enzymes, β-ketoacyl-acyl carrier protein (ACP) reductase and enoyl-ACP reductase, catalyze two of the reactions that constitute the core four-reaction cycle of the FAS system, which iteratively elongates the acyl chain by two carbon atoms per cycle. The third enzyme, malonyl-coenzyme A:ACP transacylase, catalyzes the reaction that loads the mtFAS system with substrate by malonylating the phosphopantetheinyl cofactor of ACP. GFP fusion experiments revealed that the these enzymes localize to both chloroplasts and mitochondria. This localization was validated by characterization of mutant alleles, which were rescued by transgenes expressing enzyme variants that were retargeted only to plastids or only to mitochondria. The singular retargeting of these proteins to plastids rescued the embryo lethality associated with disruption of the essential ptFAS system, but these rescued plants displayed phenotypes typical of the lack of mtFAS function, including reduced lipoylation of the H subunit of the glycine decarboxylase complex, hyperaccumulation of glycine, and reduced growth. However, these latter traits were reversible in an elevated-CO2 atmosphere, which suppresses mtFAS-associated photorespiration-dependent chemotypes. Sharing enzymatic components between mtFAS and ptFAS systems constrains the evolution of these nonredundant fatty acid biosynthetic machineries.
© 2020 American Society of Plant Biologists. All Rights Reserved.
Figures







Similar articles
-
Discovery and Characterization of the 3-Hydroxyacyl-ACP Dehydratase Component of the Plant Mitochondrial Fatty Acid Synthase System.Plant Physiol. 2017 Apr;173(4):2010-2028. doi: 10.1104/pp.16.01732. Epub 2017 Feb 15. Plant Physiol. 2017. PMID: 28202596 Free PMC article.
-
A phosphopantetheinyl transferase that is essential for mitochondrial fatty acid biosynthesis.Plant J. 2015 Nov;84(4):718-32. doi: 10.1111/tpj.13034. Plant J. 2015. PMID: 26402847
-
Mitochondrial Fatty Acid Synthase Utilizes Multiple Acyl Carrier Protein Isoforms.Plant Physiol. 2020 Jun;183(2):547-557. doi: 10.1104/pp.19.01468. Epub 2020 Feb 24. Plant Physiol. 2020. PMID: 32094306 Free PMC article.
-
Extending the story of very-long-chain fatty acid elongation.Plant Sci. 2013 Sep;210:93-107. doi: 10.1016/j.plantsci.2013.05.008. Epub 2013 May 22. Plant Sci. 2013. PMID: 23849117 Review.
-
Structural and functional organization of the animal fatty acid synthase.Prog Lipid Res. 2003 Jul;42(4):289-317. doi: 10.1016/s0163-7827(02)00067-x. Prog Lipid Res. 2003. PMID: 12689621 Review.
Cited by
-
Protein lipoylation in mitochondria requires Fe-S cluster assembly factors NFU4 and NFU5.Plant Physiol. 2022 Feb 4;188(2):997-1013. doi: 10.1093/plphys/kiab501. Plant Physiol. 2022. PMID: 34718778 Free PMC article.
-
Co-regulation of mitochondrial and chloroplast function: Molecular components and mechanisms.Plant Commun. 2023 Jan 9;4(1):100496. doi: 10.1016/j.xplc.2022.100496. Epub 2022 Nov 26. Plant Commun. 2023. PMID: 36435968 Free PMC article. Review.
-
Landscape of the oncogenic role of fatty acid synthase in human tumors.Aging (Albany NY). 2021 Dec 8;13(23):25106-25137. doi: 10.18632/aging.203730. Epub 2021 Dec 8. Aging (Albany NY). 2021. PMID: 34879004 Free PMC article.
-
A Multi-Year, Multi-Cultivar Approach to Differential Expression Analysis of High- and Low-Protein Soybean (Glycine max).Int J Mol Sci. 2022 Dec 23;24(1):222. doi: 10.3390/ijms24010222. Int J Mol Sci. 2022. PMID: 36613666 Free PMC article.
-
The genomes of Vischeria oleaginous microalgae shed light on the molecular basis of hyper-accumulation of lipids.BMC Biol. 2023 Jun 6;21(1):133. doi: 10.1186/s12915-023-01618-x. BMC Biol. 2023. PMID: 37280620 Free PMC article.
References
-
- Babiychuk E, Müller F, Eubel H, Braun HP, Frentzen M, Kushnir S(2003) Arabidopsis phosphatidylglycerophosphate synthase 1 is essential for chloroplast differentiation, but is dispensable for mitochondrial function. Plant J 33: 899–909 - PubMed
-
- Bahaji A, Ovecka M, Bárány I, Risueño MC, Muñoz FJ, Baroja-Fernández E, Montero M, Li J, Hidalgo M, Sesma MT, et al. (2011) Dual targeting to mitochondria and plastids of AtBT1 and ZmBT1, two members of the mitochondrial carrier family. Plant Cell Physiol 52: 597–609 - PubMed
-
- Beisson F, Koo AJ, Ruuska S, Schwender J, Pollard M, Thelen JJ, Paddock T, Salas JJ, Savage L, Milcamps A, et al. (2003) Arabidopsis genes involved in acyl lipid metabolism: A 2003 census of the candidates, a study of the distribution of expressed sequence tags in organs, and a web-based database. Plant Physiol 132: 681–697 - PMC - PubMed
-
- Berglund AK, Pujol C, Duchene AM, Glaser E(2009a) Defining the determinants for dual targeting of amino acyl-tRNA synthetases to mitochondria and chloroplasts. J Mol Biol 393: 803–814 - PubMed
-
- Berglund AK, Spånning E, Biverståhl H, Maddalo G, Tellgren-Roth C, Mäler L, Glaser E(2009b) Dual targeting to mitochondria and chloroplasts: Characterization of Thr-tRNA synthetase targeting peptide. Mol Plant 2: 1298–1309 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

