The polymorphism rs975484 in the protein arginine methyltransferase 1 gene modulates expression of immune checkpoint genes in hepatocellular carcinoma
- PMID: 32245889
- PMCID: PMC7242710
- DOI: 10.1074/jbc.RA120.013401
The polymorphism rs975484 in the protein arginine methyltransferase 1 gene modulates expression of immune checkpoint genes in hepatocellular carcinoma
Abstract
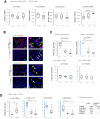
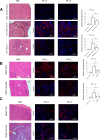
Protein arginine methyltransferase 1 (PRMT1) is a key regulator of hepatic immune responses. Recently, we reported that PRMT1 regulates the tumor immune response in hepatocellular carcinoma (HCC). Here we found that PRMT1 expression in human HCC correlates with that of programmed cell death 1 ligand 1 (PD-L1), PD-L2, and other checkpoint genes. PRMT1 deletion in mice reduced PD-L1 and PD-L2 expression in tumors and reduced the efficiency of PD-1 antibody treatment in a diethylnitrosamine-induced HCC mouse model, suggesting that PRMT1 regulates the hepatic immune checkpoint. Mice had reduced PD-L1 and PD-L2 expression when PRMT1 was specifically deleted in tumor cells or macrophages, but PRMT1 deletion in dendritic cells did not alter PD-L1 and PD-L2 expression. rs975484 is a common polymorphism in the human PRMT1 gene promoter, and we found that it alters PRMT1 expression in blood monocytes and tumor-associated macrophages in human HCC. PRMT1 expression was higher in individuals with a GG genotype than in individuals with a CC genotype, and heterozygous carriers had intermediate expression. Luciferase reporter assays indicated that this differential expression is due to an extra C/EBPβ-binding site in the PRMT1 promoter of individuals carrying the minor G allele. The rs975484 genotype also correlated with PRMT1 target expression in HCC. Individuals with the GG genotype had significantly higher levels of the PRMT1 targets PD-L1, PD-L2, and VISTA than those with the CC genotype. We conclude that PRMT1 critically controls immune checkpoints in mice and humans and that the PRMT1 polymorphism rs975484 affects checkpoint gene expression in HCC.
Keywords: CCAAT-enhancer-binding protein (C/EBP); PD-L2; SNP; checkpoint control; inflammation; macrophage; programmed cell death 1 ligand 1 (PD-L1); protein arginine methyltransferase 1 (PRMT1); rs975484; tumor immunology.
© 2020 Schonfeld et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

