β-Catenin and FGFR2 regulate postnatal rosette-based adrenocortical morphogenesis
- PMID: 32245949
- PMCID: PMC7125176
- DOI: 10.1038/s41467-020-15332-7
β-Catenin and FGFR2 regulate postnatal rosette-based adrenocortical morphogenesis
Abstract
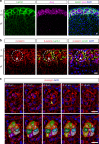
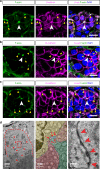
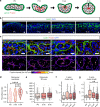
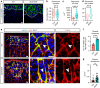
Rosettes are widely used in epithelial morphogenesis during embryonic development and organogenesis. However, their role in postnatal development and adult tissue maintenance remains largely unknown. Here, we show zona glomerulosa cells in the adult adrenal cortex organize into rosettes through adherens junction-mediated constriction, and that rosette formation underlies the maturation of adrenal glomerular structure postnatally. Using genetic mouse models, we show loss of β-catenin results in disrupted adherens junctions, reduced rosette number, and dysmorphic glomeruli, whereas β-catenin stabilization leads to increased adherens junction abundance, more rosettes, and glomerular expansion. Furthermore, we uncover numerous known regulators of epithelial morphogenesis enriched in β-catenin-stabilized adrenals. Among these genes, we show Fgfr2 is required for adrenal rosette formation by regulating adherens junction abundance and aggregation. Together, our data provide an example of rosette-mediated postnatal tissue morphogenesis and a framework for studying the role of rosettes in adult zona glomerulosa tissue maintenance and function.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

