Milk lactoperoxidase decreases ID1 and ID3 expression in human oral squamous cell carcinoma cell lines
- PMID: 32246075
- PMCID: PMC7125221
- DOI: 10.1038/s41598-020-62390-4
Milk lactoperoxidase decreases ID1 and ID3 expression in human oral squamous cell carcinoma cell lines
Abstract
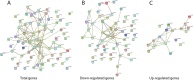
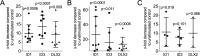
Milk consumption may modify the risk of squamous cell carcinoma. The role of milk to modulate the gene expression in oral squamous cell carcinoma cells has not been investigated so far. Here, HSC2 oral squamous carcinoma cells were exposed to an aqueous fraction of human milk and a whole-genome array was performed. Among the genes that were significantly reduced by human and cow milk were the DNA-binding protein inhibitor 1 (ID1), ID3 and Distal-Less Homeobox 2 (DLX2) in HSC2 cells. Also, in TR146 oral squamous carcinoma cells, there was a tendency towards a decreased gene expression. Upon size fractionation, lactoperoxidase but not lactoferrin and osteopontin was identified to reduce ID1 and ID3 in HSC2 cells. Dairy products and hypoallergenic infant formula failed to decrease the respective genes. These data suggest that milk can reduce the expression of transcription factors in oral squamous carcinoma cells.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Micellar Casein and Whey Powder Hold a TGF-β Activity and Regulate ID Genes In Vitro.Molecules. 2021 Jan 19;26(2):507. doi: 10.3390/molecules26020507. Molecules. 2021. PMID: 33477984 Free PMC article.
-
[Expression of Id1 and Id3 in endometrial carcinoma and their roles in regulating biological behaviors of endometrial carcinoma cells in vitro].Nan Fang Yi Ke Da Xue Xue Bao. 2013 Jun;33(6):812-8. Nan Fang Yi Ke Da Xue Xue Bao. 2013. PMID: 23803189 Chinese.
-
Targeting Id1 and Id3 by a specific peptide aptamer induces E-box promoter activity, cell cycle arrest, and apoptosis in breast cancer cells.Breast Cancer Res Treat. 2010 Dec;124(3):623-33. doi: 10.1007/s10549-010-0810-6. Epub 2010 Feb 27. Breast Cancer Res Treat. 2010. PMID: 20191379
-
Id1/Id3 knockdown inhibits metastatic potential of pancreatic cancer.J Surg Res. 2010 Jun 1;161(1):76-82. doi: 10.1016/j.jss.2008.10.031. Epub 2008 Nov 29. J Surg Res. 2010. PMID: 19515385
-
Eldecalcitol (ED-71), an analog of 1α,25-dihydroxyvitamin D3 as a potential anti-cancer agent for oral squamous cell carcinomas.J Steroid Biochem Mol Biol. 2016 Nov;164:79-84. doi: 10.1016/j.jsbmb.2015.09.043. Epub 2015 Oct 9. J Steroid Biochem Mol Biol. 2016. PMID: 26444325 Review.
Cited by
-
Influence of dairy products consumption on oral cancer risk: A meta-analysis.J Dent Res Dent Clin Dent Prospects. 2023 Winter;17(1):1-7. doi: 10.34172/joddd.2023.36851. Epub 2023 Apr 3. J Dent Res Dent Clin Dent Prospects. 2023. PMID: 37650016 Free PMC article. Review.
-
Micellar Casein and Whey Powder Hold a TGF-β Activity and Regulate ID Genes In Vitro.Molecules. 2021 Jan 19;26(2):507. doi: 10.3390/molecules26020507. Molecules. 2021. PMID: 33477984 Free PMC article.
-
Effect of comprehensive nursing on the appearance and recovery effect of oral squamous cell carcinoma patients.Am J Transl Res. 2021 May 15;13(5):5519-5525. eCollection 2021. Am J Transl Res. 2021. PMID: 34150152 Free PMC article.
-
Milk-Derived Proteins and Peptides in Head and Neck Carcinoma Treatment.Biomolecules. 2022 Feb 10;12(2):290. doi: 10.3390/biom12020290. Biomolecules. 2022. PMID: 35204791 Free PMC article.
-
RNAseq of TGF-β receptor type I kinase-dependent genes in oral fibroblast exposed to milk.BMC Oral Health. 2021 Nov 16;21(1):581. doi: 10.1186/s12903-021-01913-5. BMC Oral Health. 2021. PMID: 34789212 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

