Lin28 reprograms inner ear glia to a neuronal fate
- PMID: 32246510
- PMCID: PMC10908373
- DOI: 10.1002/stem.3181
Lin28 reprograms inner ear glia to a neuronal fate
Abstract
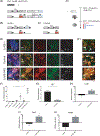
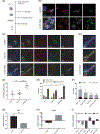
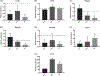
Sensorineural hearing loss is irreversible and can be caused by loss of auditory neurons. Regeneration of neural cells from endogenous cells may offer a future tool to restore the auditory circuit and to enhance the performance of implantable hearing devices. Neurons and glial cells in the peripheral nervous system are closely related and originate from a common progenitor. Prior work in our lab indicated that in the early postnatal mouse inner ear, proteolipid protein 1 (Plp1) expressing glial cells could act as progenitor cells for neurons in vitro. Here, we used a transgenic mouse model to transiently overexpress Lin28, a neural stem cell regulator, in Plp1-positive glial cells. Lin28 promoted proliferation and conversion of auditory glial cells into neurons in vitro. To study the effects of Lin28 on endogenous glial cells after loss of auditory neurons in vivo, we produced a model of auditory neuropathy by selectively damaging auditory neurons with ouabain. After neural damage was confirmed by the auditory brainstem response, we briefly upregulated the Lin28 in Plp1-expressing inner ear glial cells. One month later, we analyzed the cochlea for neural marker expression by quantitative RT-PCR and immunohistochemistry. We found that transient Lin28 overexpression in Plp1-expressing glial cells induced expression of neural stem cell markers and subsequent conversion into neurons. This suggests the potential for inner ear glia to be converted into neurons as a regeneration therapy for neural replacement in auditory neuropathy.
Keywords: glial conversion; inner ear; mouse models; neural stem cells; regeneration.
©AlphaMed Press 2020.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
Figures

Similar articles
-
Sox2 up-regulation and glial cell proliferation following degeneration of spiral ganglion neurons in the adult mouse inner ear.J Assoc Res Otolaryngol. 2011 Apr;12(2):151-71. doi: 10.1007/s10162-010-0244-1. J Assoc Res Otolaryngol. 2011. PMID: 21061038 Free PMC article.
-
Fate of neural stem cells grafted into injured inner ears of mice.Neuroreport. 2003 Sep 15;14(13):1677-81. doi: 10.1097/00001756-200309150-00004. Neuroreport. 2003. PMID: 14512836
-
Morin hydrate promotes inner ear neural stem cell survival and differentiation and protects cochlea against neuronal hearing loss.J Cell Mol Med. 2017 Mar;21(3):600-608. doi: 10.1111/jcmm.13005. Epub 2016 Oct 6. J Cell Mol Med. 2017. PMID: 27709784 Free PMC article.
-
The biological strategies for hearing re-establishment based on the stem/progenitor cells.Neurosci Lett. 2019 Oct 15;711:134406. doi: 10.1016/j.neulet.2019.134406. Epub 2019 Aug 1. Neurosci Lett. 2019. PMID: 31377244 Review.
-
Stem cells for the replacement of inner ear neurons and hair cells.Int J Dev Biol. 2007;51(6-7):655-61. doi: 10.1387/ijdb.072372rm. Int J Dev Biol. 2007. PMID: 17891724 Review.
Cited by
-
Cochlear Sox2+ Glial Cells Are Potent Progenitors for Spiral Ganglion Neuron Reprogramming Induced by Small Molecules.Front Cell Dev Biol. 2021 Sep 21;9:728352. doi: 10.3389/fcell.2021.728352. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34621745 Free PMC article.
-
A human induced pluripotent stem cell-based modular platform to challenge sensorineural hearing loss.Stem Cells. 2021 Jun;39(6):697-706. doi: 10.1002/stem.3346. Epub 2021 Feb 8. Stem Cells. 2021. PMID: 33522002 Free PMC article. Review.
-
LIN28A: A multifunctional versatile molecule with future therapeutic potential.World J Biol Chem. 2022 Mar 27;13(2):35-46. doi: 10.4331/wjbc.v13.i2.35. World J Biol Chem. 2022. PMID: 35432768 Free PMC article. Review.
-
Growth factors with valproic acid restore injury-impaired hearing by promoting neuronal regeneration.JCI Insight. 2021 Nov 22;6(22):e139171. doi: 10.1172/jci.insight.139171. JCI Insight. 2021. PMID: 34806649 Free PMC article.
-
Wnt signalling facilitates neuronal differentiation of cochlear Frizzled10-positive cells in mouse cochlea via glypican 6 modulation.Cell Commun Signal. 2025 Jan 27;23(1):50. doi: 10.1186/s12964-025-02039-9. Cell Commun Signal. 2025. PMID: 39871249 Free PMC article.
References
-
- Nadol JB Jr, Young YS, Glynn RJ. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1989;98(6):411–416. - PubMed
-
- Nadol JB Jr. Patterns of neural degeneration in the human cochlea and auditory nerve: implications for cochlear implantation. Otolaryngol Head Neck Surg. 1997;117(3 Pt 1):220–228. - PubMed
-
- Moller AR. History of cochlear implants and auditory brainstem implants. Adv Otorhinolaryngol. 2006;64:1–10. - PubMed
-
- Lustig LR, Yeagle J, Driscoll CL, Blevins N, Francis H, Niparko JK. Cochlear implantation in patients with neurofibromatosis type 2 and bilateral vestibular schwannoma. Otol Neurotol. 2006;27(4):512–518. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

