Spinal genesis of Mayer waves
- PMID: 32246623
- PMCID: PMC7513982
- DOI: 10.4103/1673-5374.280306
Spinal genesis of Mayer waves
Retraction in
-
Retraction: Spinal genesis of Mayer waves.Neural Regen Res. 2022 May;17(5):947. doi: 10.4103/1673-5374.295351. Neural Regen Res. 2022. PMID: 34558546 Free PMC article.
Abstract
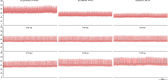
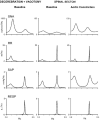
Variability in cardiovascular spectra was first described by Stephan Hales in 1733. Traube and Hering initially noted respirophasic variation of the arterial pressure waveform in 1865 and Sigmund Mayer noted a lower frequency oscillation of the same in anesthetized rabbits in 1876. Very low frequency oscillations were noted by Barcroft and Nisimaru in 1932, likely representing vasogenic autorhythmicity. While the origins of Traube Hering and very low frequency oscillatory variability in cardiovascular spectra are well described, genesis mechanisms and functional significance of Mayer waves remain in controversy. Various theories have posited baroreflex and central supraspinal mechanisms for genesis of Mayer waves. Several studies have demonstrated the persistence of Mayer waves following high cervical transection, indicating a spinal capacity for genesis of these oscillations. We suggest a general tendency for central sympathetic neurons to oscillate at the Mayer wave frequency, the presence of multiple Mayer wave oscillators throughout the brainstem and spinal cord, and possible contemporaneous genesis by baroreflex and vasomotor mechanisms.
Keywords: Mayer waves; central; cervical; genesis; origins; spinal cord; sympathogenesis; transection.
Conflict of interest statement
None
Figures



References
-
- Anderson B, Kenny BA, Neil E. Role of the chemoreceptors of the carotid and aortic regions in the production of the Mayer waves. Acta Physiol Scand. 1950;20:203. - PubMed
-
- Aoki M, Mori S, Kawahara K, Watanabe H, Ebata N. Generation of spontaneous respiratory rhythm in high spinal cats. Brain Res. 1980;202:51–63. - PubMed
-
- Baldridge BR, Burgess DE, Zimmerman EE, Carroll JJ, Sprinkle AG, Speakman RO, Li SG, Brown DR, Taylor RF, Dworkin S, Randall DC. Heart rate-arterial blood pressure relationship in conscious rat before vs. after spinal cord transection. Am J Physiol Regul Integr Comp Physiol. 2002;283:R748–756. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

