Flavin-Dependent Monooxygenases NotI and NotI' Mediate Spiro-Oxindole Formation in Biosynthesis of the Notoamides
- PMID: 32246875
- PMCID: PMC7483341
- DOI: 10.1002/cbic.202000004
Flavin-Dependent Monooxygenases NotI and NotI' Mediate Spiro-Oxindole Formation in Biosynthesis of the Notoamides
Abstract
The fungal indole alkaloids are a unique class of complex molecules that have a characteristic bicyclo[2.2.2]diazaoctane ring and frequently contain a spiro-oxindole moiety. While various strains produce these compounds, an intriguing case involves the formation of individual antipodes by two unique species of fungi in the generation of the potent anticancer agents (+)- and (-)-notoamide A. NotI and NotI' have been characterized as flavin-dependent monooxygenases that catalyze epoxidation and semi-pinacol rearrangement to form the spiro-oxindole center within these molecules. This work elucidates a key step in the biosynthesis of the notoamides and provides an evolutionary hypothesis regarding a common ancestor for production of enantiopure notoamides.
Keywords: alkaloids; biosynthesis; flavin monooxygenase; notoamide; spirocycle.
© 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures
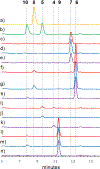
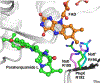


References
-
- Birch AJ, Wright JJ, Chem Commun 1969, 644–645;
- Steyn PS, Tetrahedron 1973, 29, 107–120;
- Polonsky J, Merrien MA, Prange T, Pascard C, Moreau S, J.C.S. Chem. Comm 1980, 601–602;
- Prangé T, Billion MA, Vuilhorgne M, Pascard C, Polonsky J, Moreau S, Tetrahedron Lett 1981, 22, 1977–1980;
- Yamazaki M, Okuyama E, Kobayashi M, Inoue H, Tetrahedron Lett 1981, 22, 135–136;
- Ondeyka JG, Goegelman RT, Schaeffer JM, Kelemen L, Zitano L, J Antibiot 1990, 43, 1375–1379; - PubMed
- Liesch JM, Wichmann CF, J Antibiot 1990, 43, 1380–1386; - PubMed
- Blanchflower SE, Banks RM, Everett JR, Manger BR, Reading C, J Antibiot 1991, 44, 492–497; - PubMed
- Blanchflower SE, Banks RM, Everett JR, Reading C, J Antibiot 1993, 46, 1355–1363; - PubMed
- Whyte AC, Gloer JB, J Nat Prod 1996, 59, 1093–1095; - PubMed
- Hayashi H, Nishimoto Y, Nozaki H, Tetrahedron Lett 1997, 38, 5655–5658;
- Sugie Y, Dekker KA, Hirai H, Ichiba T, Ishiguro M, Shiomi Y, Sugiura A, Brennan L, Duignan J, Huang LH, Sutcliffe J, Kojima Y, J Antibiot 2001, 54, 1060–1065; - PubMed
- Qian-Cutrone JF, Huang S, Shu YZ, Vyas D, Fairchild C, Menendez A, Krampitz K, Dalterio R, Klohr SE, Gao Q, J Am Chem Soc 2002, 124, 14556–14557; - PubMed
- Martínez-Luis S, Rodríguez R, Acevedo L, González MC, Lira-Rocha A, Mata R, Tetrahedron 2006, 62, 1817–1822.
-
- Adams LA, Gray CR, Williams RM, Tetrahedron Lett 2004, 45, 4489–4493;
- Adams LA, Valente MWN, Williams RM, Tetrahedron 2006, 62, 5195–5200;
- Greshock TJ, Grubbs AW, Williams RM, Tetrahedron 2007, 63, 6124–6130; - PMC - PubMed
- Greshock TJ, Grubbs AW, Tsukamoto S, Williams RM, Angew Chem Int Edit 2007, 46, 2262–2265; - PubMed
- Miller KA, Welch TR, Greshock TJ, Ding YS, Sherman DH, Williams RM, J Org Chem 2008, 73, 3116–3119; - PMC - PubMed
- Miller KA, Williams RM, Chem Soc Rev 2009, 38, 3160–3174; - PMC - PubMed
- Miller KA, Tsukamoto S, Williams RM, Nat Chem 2009, 1, 63–68; - PMC - PubMed
- Mukai K, de Sant’Ana DP, Hirooka Y, Mercado-Marin EV, Stephens DE, Kou KGM, Richter SC, Kelley N, Sarpong R, Nat Chem 2018, 10, 38–44. - PMC - PubMed
-
- Kato H, Yoshida T, Tokue T, Nojiri Y, Hirota H, Ohta T, Williams RM, Tsukamoto S, Angew Chem Int Edit 2007, 46, 2254–2256; - PubMed
- Tsukamoto S, Kato H, Samizo M, Nojiri Y, Onuki H, Hirota H, Ohta T, J Nat Prod 2008, 71, 2064–2067; - PubMed
- Tsukamoto S, Kato H, Greshock TJ, Hirota H, Ohta T, Williams RM, J Am Chem Soc 2009, 131, 3834–3835. - PMC - PubMed
-
- Tsuda M, Kasai Y, Komatsu K, Sone T, Tanaka M, Mikami Y, Kobayashi J, Org Lett 2004, 6, 3087–3089. - PubMed
-
- Li SM, Nat Prod Rep 2010, 27, 57–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

