Pharmacodynamic effects of vaporized and oral cannabidiol (CBD) and vaporized CBD-dominant cannabis in infrequent cannabis users
- PMID: 32247649
- PMCID: PMC7414803
- DOI: 10.1016/j.drugalcdep.2020.107937
Pharmacodynamic effects of vaporized and oral cannabidiol (CBD) and vaporized CBD-dominant cannabis in infrequent cannabis users
Abstract
Introduction: The use and availability of oral and inhalable products containing cannabidiol (CBD) as the principal constituent has increased with expanded cannabis/hemp legalization. However, few controlled clinical laboratory studies have evaluated the pharmacodynamic effects of oral or vaporized CBD or CBD-dominant cannabis.
Methods: Eighteen healthy adults (9 men; 9 women) completed four, double-blind, double-dummy, drug administration sessions. Sessions were separated by ≥1 week and included self-administration of 100 mg oral CBD, 100 mg vaporized CBD, vaporized CBD-dominant cannabis (100 mg CBD; 3.7 mg THC), and placebo. Study outcomes included: subjective drug effects, vital signs, cognitive/psychomotor performance, and whole blood THC and CBD concentrations.
Results: Vaporized CBD and CBD-dominant cannabis increased ratings on several subjective items (e.g., Like Drug Effect) relative to placebo. Subjective effects did not differ between oral CBD and placebo and were generally higher for CBD-dominant cannabis compared to vaporized CBD. CBD did not increase ratings for several items typically associated with acute cannabis/THC exposure (e.g., Paranoid). Women reported qualitatively higher ratings for Pleasant Drug Effect than men after vaporized CBD and CBD-dominant cannabis use. CBD-dominant cannabis increased heart rate compared to placebo. Cognitive/psychomotor impairment was not observed in any drug condition.
Conclusions: Vaporized CBD and CBD-dominant cannabis produced discriminable subjective drug effects, which were sometimes stronger in women, but did not produce cognitive/psychomotor impairment. Subjective effects of oral CBD did not differ from placebo. Future research should further elucidate the subjective effects of various types of CBD products (e.g., inhaled, oral, topical), which appear to be distinct from THC-dominant products.
Keywords: Cannabidiol; Cannabis; Pharmacodynamics; THC; Vaporizer.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Dr. Vandrey has served as a consultant or received honoraria from Zynerba Pharmaceuticals, FSD Pharma, and Canopy Health Innovations Inc.
Figures

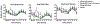
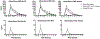
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

