Extracellular matrix dynamics in tubulogenesis
- PMID: 32247774
- PMCID: PMC7269847
- DOI: 10.1016/j.cellsig.2020.109619
Extracellular matrix dynamics in tubulogenesis
Abstract
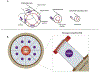
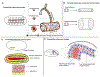
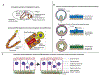
Biological tubes form in a variety of shapes and sizes. Tubular topology of cells and tissues is a widely recognizable histological feature of multicellular life. Fluid secretion, storage, transport, absorption, exchange, and elimination-processes central to metazoans-hinge on the exquisite tubular architectures of cells, tissues, and organs. In general, the apparent structural and functional complexity of tubular tissues and organs parallels the architectural and biophysical properties of their constitution, i.e., cells and the extracellular matrix (ECM). Together, cellular and ECM dynamics determine the developmental trajectory, topological characteristics, and functional efficacy of biological tubes. In this review of tubulogenesis, we highlight the multifarious roles of ECM dynamics-the less recognized and poorly understood morphogenetic counterpart of cellular dynamics. The ECM is a dynamic, tripartite composite spanning the luminal, abluminal, and interstitial space within the tubulogenic realm. The critical role of ECM dynamics in the determination of shape, size, and function of tubes is evinced by developmental studies across multiple levels-from morphological through molecular-in model tubular organs.
Keywords: Branching morphogenesis; Development; Embryo; Extracellular matrix; Lumen; Tubulogenesis.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no competing financial interests.
Figures
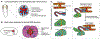

References
-
- Schmidt-Rhaesa A, The Evoution of Organ Systems, Oxford University Press Inc,, New York, 2007.
-
- West GB, Brown JH, Enquist BJ, The fourth dimension of life: fractal geometry and allometric scaling of organisms, Science (New York, N.Y.) 284(5420) (1999) 1677–9. - PubMed
-
- Cool J, DeFalco T, Capel B, Testis formation in the fetal mouse: dynamic and complex de novo tubulogenesis, Wiley interdisciplinary reviews. Developmental biology 1(6) (2012) 847–59. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

